FDA: Page 3
-
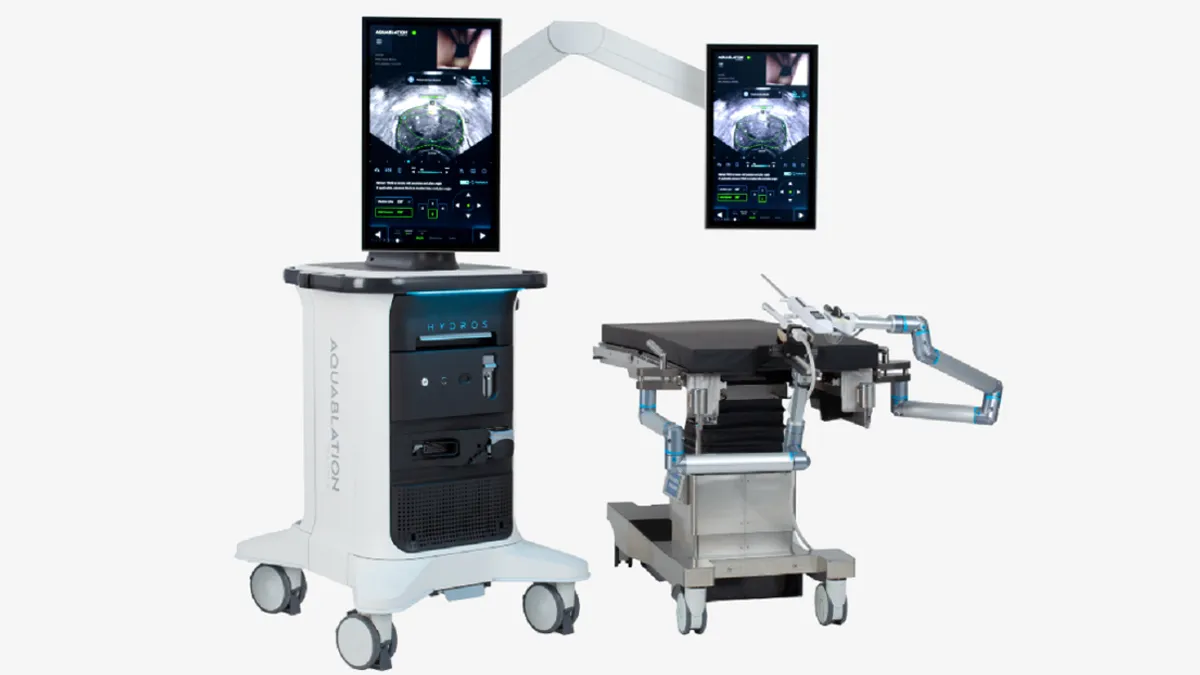
Procept wins FDA clearance for updated prostate surgery robot
Procept Biorobotics has added AI capabilities and switched to a single-use scope to support mass-market adoption.
By Nick Paul Taylor • Aug. 22, 2024 -
FDA defends Shuren’s tenure after report raises ethics concerns
The FDA backed Jeff Shuren, the agency’s former device leader, amid questions raised by The New York Times. Still, a spokesperson said the FDA has advised Shuren to take greater caution in managing recusal obligations.
By Susan Kelly • Aug. 22, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineMedical device safety in spotlight after high profile recalls
From Philips’ massive recall of respiratory devices to ongoing health risks with breast implants, medical devices tied to patient harm have put a focus on product safety.
By MedTech Dive staff -

 "Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
"Government Accountability Office Building" by kafka4prez is licensed under CC BY-SA 2.0
FDA’s plan to expand device surveillance faces challenges, GAO finds
Funding issues and limited use of unique device identifiers are hurdles to expanding an FDA system to monitor medical device safety, the federal watchdog said.
By Nick Paul Taylor • Aug. 21, 2024 -
FDA OKs first at-home syphilis test
NowDiagnostics’ over-the-counter test can return results in 15 minutes with a single drop of blood.
By Ricky Zipp • Aug. 19, 2024 -
Boston Scientific resubmits Silk Road merger filing, giving FTC more review time
A Boston Scientific spokesperson said the company still expects to close its $1.26 billion purchase of Silk Road Medical in the second half of the year.
By Ricky Zipp • Aug. 16, 2024 -

 Erin Scott / U.S. White House. Retrieved from Flickr.
Erin Scott / U.S. White House. Retrieved from Flickr.
Biden pledges $150M for medical tech to improve cancer surgery
Awards include funding for microscopy and imaging tools to help surgeons ensure all cancer is removed during a procedure.
By Elise Reuter • Aug. 16, 2024 -
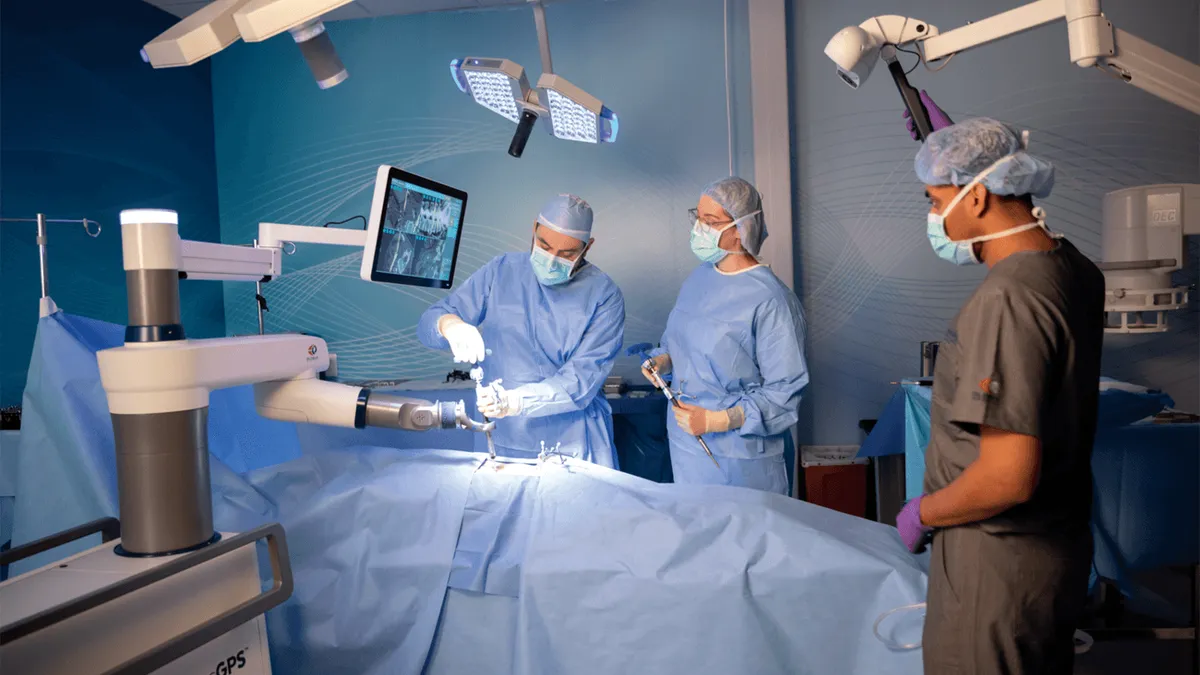
 Retrieved from Globus Medical on August 14, 2024
Retrieved from Globus Medical on August 14, 2024
Globus receives warning letter over spine navigation robot
The FDA said Globus delayed reporting complaints about misplaced screws, despite information that “reasonably suggests” the Excelsius GPS system malfunctioned.
By Nick Paul Taylor • Aug. 14, 2024 -
Medtronic wins FDA approval for asleep deep brain stimulation
Medtronic said Monday it is the first company to receive FDA approval to offer DBS surgery while a patient is asleep or awake.
By Nick Paul Taylor • Aug. 13, 2024 -
CMS finalizes notice on Medicare coverage for breakthrough devices
Through the new pathway, called Transitional Coverage for Emerging Technologies, the CMS will consider five medical device candidates yearly for national coverage.
By Elise Reuter • Aug. 8, 2024 -
FDA seeks feedback on health equity for medical devices
The agency plans to develop a framework for when devices should be evaluated in diverse populations to support marketing authorization.
By Nick Paul Taylor • Aug. 6, 2024 -
Labcorp wins FDA OK for pan-solid tumor liquid biopsy
Shakti Ramkissoon, Labcorp’s medical lead for oncology, said the test could allow laboratories and oncologists to profile solid tumors when tissue samples are limited or unavailable.
By Nick Paul Taylor • Aug. 6, 2024 -
Inspire wins FDA approval for obstructive sleep apnea neurostimulator therapy
Inspire plans to start a soft launch of the device late in 2024 and move into a full release in 2025.
By Nick Paul Taylor • Aug. 5, 2024 -
Experts fear patient harm from FDA’s lab developed test rule
Decreased access to diagnostic tests, worsening patient care and practice closures are among the concerns the AMA and others have raised as the regulation takes effect.
By Susan Kelly • Aug. 5, 2024 -
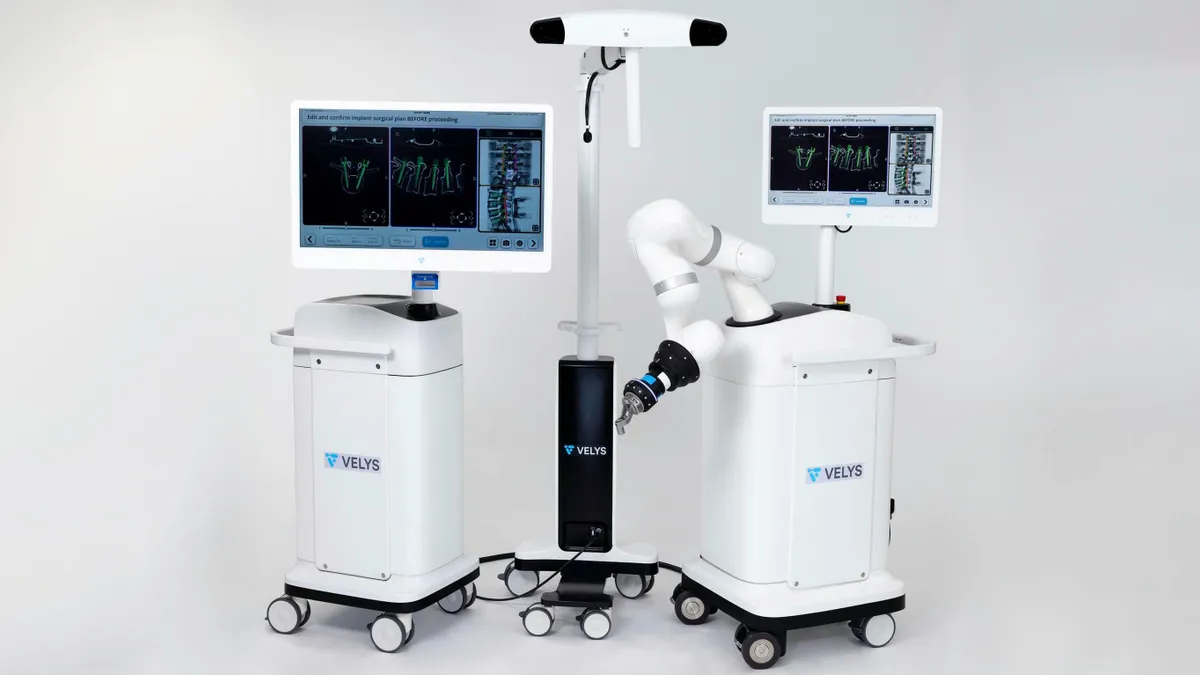
J&J launches Velys Spine surgical robotics and navigation platform
With its Velys surgical robotics portfolio, J&J aims to take market share from competitors Stryker and Zimmer Biomet.
By Nick Paul Taylor • Aug. 5, 2024 -
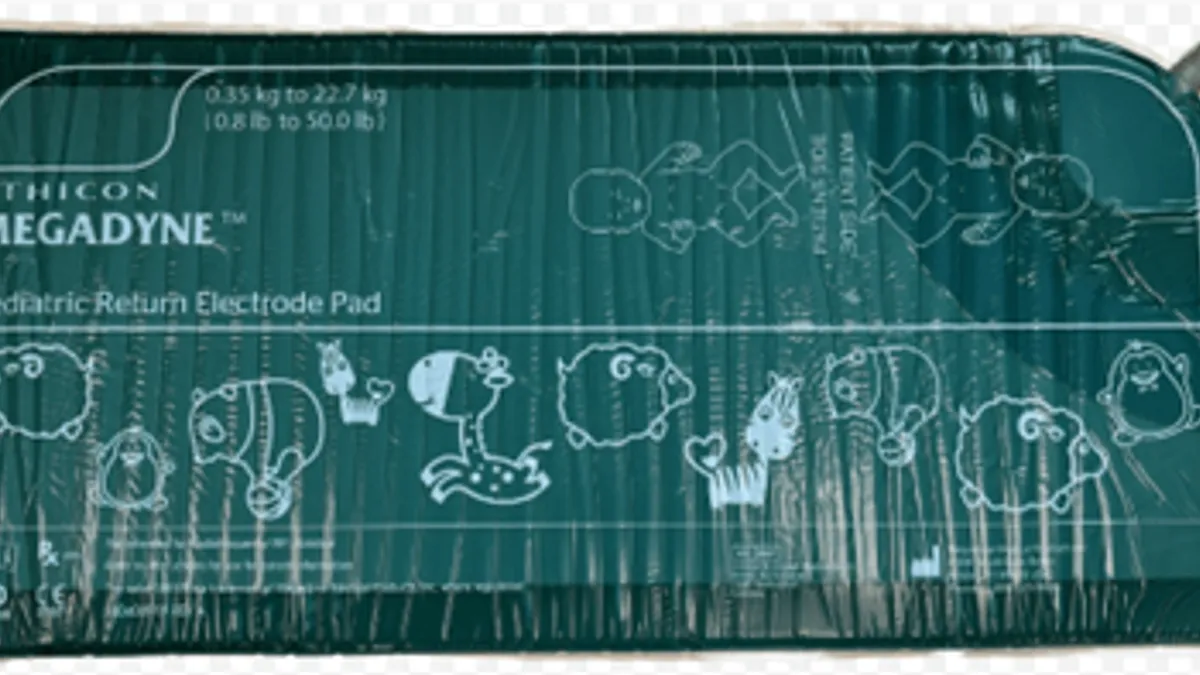
FDA, Health Canada team up to raise awareness of J&J’s Megadyne recall
Agency officials are working together to communicate the risk of burns tied to Megadyne electrode pads.
By Nick Paul Taylor • Aug. 1, 2024 -
FDA sends warning letter to defibrillator battery manufacturer
Amco makes batteries for automated external defibrillators sold by companies including Philips, Stryker and Zoll.
By Nick Paul Taylor • July 31, 2024 -
Patient shares a day in the life with diabetes at FDA’s first Home Health Hub meeting
The initiative, led by new CDRH Acting Director Michelle Tarver, is intended to improve health equity by including people’s living conditions in device design.
By Elise Reuter • July 30, 2024 -
FDA officials outline benefits of AI lifecycle management
The digital health leaders detailed a seven-stage diagram for how the healthcare industry could monitor artificial intelligence software development.
By Nick Paul Taylor • July 30, 2024 -
Guardant wins FDA approval for colon cancer blood test
Colorectal cancer screening rates could increase if the Shield test appeals to people who have avoided colonoscopies or stool-based tests, the company said.
By Susan Kelly • July 29, 2024 -
Abbott recalls Freestyle Libre 3 sensors due to incorrect glucose readings
A spokesperson for Abbott said the recall “may impact less than 1% of Libre 3 users in the U.S.”
By Nick Paul Taylor • July 26, 2024 -
CDRH Director Shuren to step down after 15 years
Michelle Tarver, deputy director for transformation, will serve as acting director of the FDA’s medical devices unit.
By Susan Kelly , Elise Reuter • July 24, 2024 -
Guardant settles allegations it violated the False Claims Act
The settlement resolves claims that an oncologist ordered significantly more tests after Guardant hired two people the physician recommended for jobs at the company.
By Nick Paul Taylor • July 23, 2024 -
FDA sends 2 warning letters after inspecting Chinese syringe manufacturers
One warning letter covers the production of plastic syringes for Cardinal Health.
By Nick Paul Taylor • July 22, 2024 -
FDA creates communication ‘super office’ at CDRH
The changes are part of a broader reorganization intended to increase organizational agility and help the agency meet user fee commitments.
By Nick Paul Taylor • July 16, 2024 -
House committee tells FDA to suspend lab developed test rule
Lawmakers said the final rule carries “the risk of greatly altering the United States’ laboratory testing infrastructure.”
By Nick Paul Taylor • July 15, 2024