The Latest
-
Vicarious Surgical receives NYSE delisting notice
The robotics company, which has taken steps to preserve cash amid the latest change of course in the development of its surgical system, got approval to move its shares to the over-the-counter market.
-
Trump tariffs
Tariff refunds: Court provides first step with liquidation order
The Court of International Trade on Wednesday directed Customs and Border Protection to remove defunct tariffs when finalizing non-liquidated entries.
-
Trump tariffs
US to hike global tariff to 15% ‘sometime this week,’ Bessent says
The Treasury Secretary also said he expects tariff rates struck down by the Supreme Court to return within the next five months, following ongoing trade investigations.
-
Stryker execs discuss Mako RPS launch at AAOS
Keith Evans, general manager of Stryker’s Mako and enabling technologies unit, said the handheld robot will be in a limited release for the first half of 2026.
-
Medtronic, GE HealthCare expand patient monitoring alliance
Expanding the long-running partnership will bring Medtronic technologies, such as the Nellcor pulse oximetry product, to more healthcare settings.
-
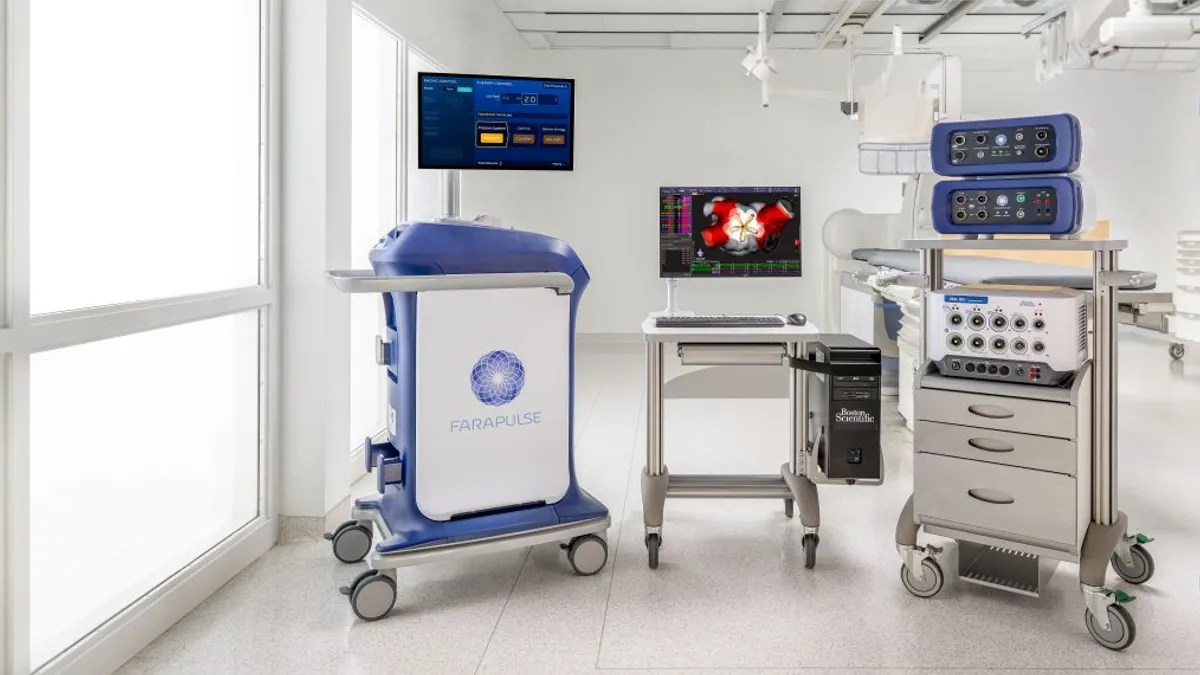
Boston Scientific gets Farapulse label expansion in Europe
With CE mark approval, the pulsed field ablation procedure becomes available to treat patients in Europe with persistent atrial fibrillation.
-
RadNet to acquire radiology AI company Gleamer
The acquisition will expand RadNet’s routine imaging portfolio and presence outside the U.S.
-
 Retrieved from Fresenius Kabi on March 03, 2026
Retrieved from Fresenius Kabi on March 03, 2026
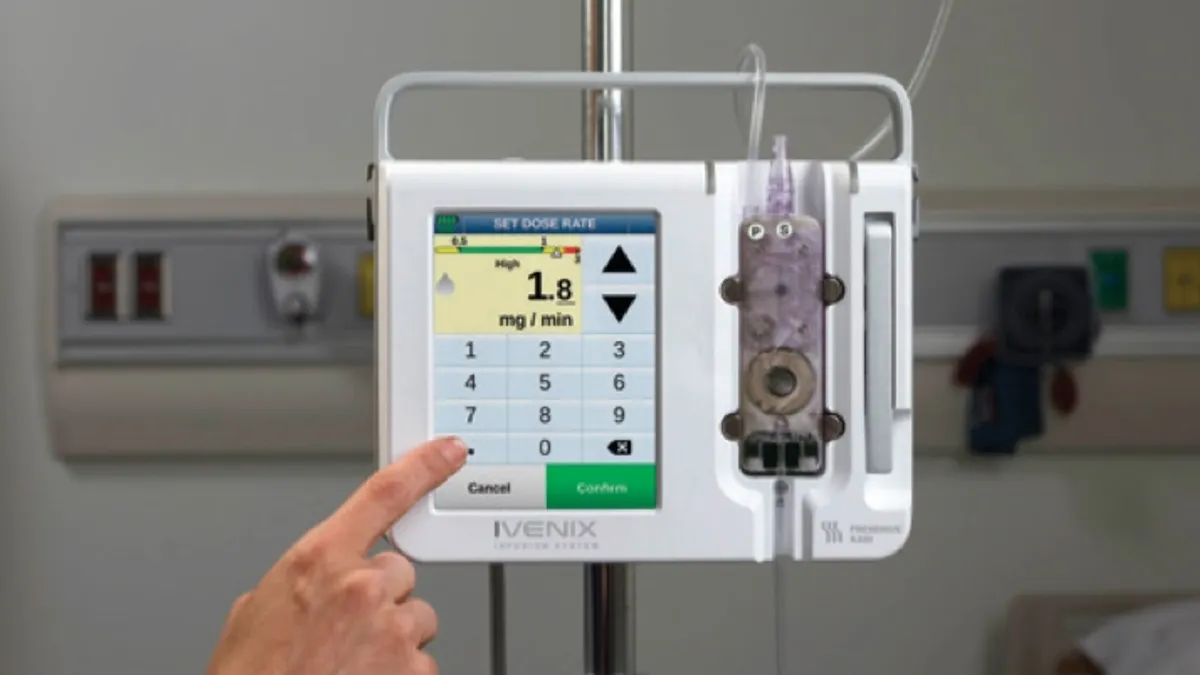
Fresenius Kabi recalls Ivenix infusion pumps over software problem
Fresenius has issued a software release to address issues that could interrupt treatment with its Ivenix large volume pumps.
-
FDA warns insulin infusion set maker Unomedical over leaks, mishandled complaints
Unomedical, which supplies diabetes tech firms including Medtronic, Tandem Diabetes Care and Beta Bionics, received more than 5,000 complaints about leaking infusion sets between 2023 and 2025.
-
Intuitive expands in Europe with purchase of distributor operations
The surgical robotics giant closed the deal, valued at about 319 million euros up front, to acquire the businesses in southern Europe.
-
Abbott wins FDA approval for updated heart failure monitoring device
The company redesigned the reader to fit in a carry-on suitcase and be easier to use day to day.
-
Trump administration targets DME suppliers in fraud crackdown
The government plans a six-month moratorium on supplier enrollment in the Medicare program to find ways to stop what it called “longstanding instances of fraud, waste, and abuse” by certain companies.