Recalls
-
Cook Medical recalls catheters over fault linked to 3 serious injuries
The company began the recall after receiving four field complaints about tip separation before and during use.
By Nick Paul Taylor • June 27, 2025 -
Medtronic recall of capsule delivery devices tied to 33 serious injuries
The Food and Drug Administration published an early alert for the recall on Tuesday. No deaths have been associated with the problem.
By Ricky Zipp • June 25, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sarah Silbiger via Getty Images
Sarah Silbiger via Getty Images Trendline
TrendlineMedical device safety in spotlight after high profile recalls
From Philips’ massive recall of respiratory devices to ongoing health risks with breast implants, medical devices tied to patient harm have put a focus on product safety.
By MedTech Dive staff -
GE Healthcare recalls Carestation devices over ventilation failure risk
The recall affects 15 Carestation models and more than 14,000 individual devices.
By Nick Paul Taylor • June 24, 2025 -
Q’Apel recalls clot removal device in response to FDA warning letter
Rather than pursuing a new regulatory pathway, Q'Apel said it is discontinuing the recalled system “as part of its strategic shift toward newer technologies.”
By Nick Paul Taylor • June 18, 2025 -
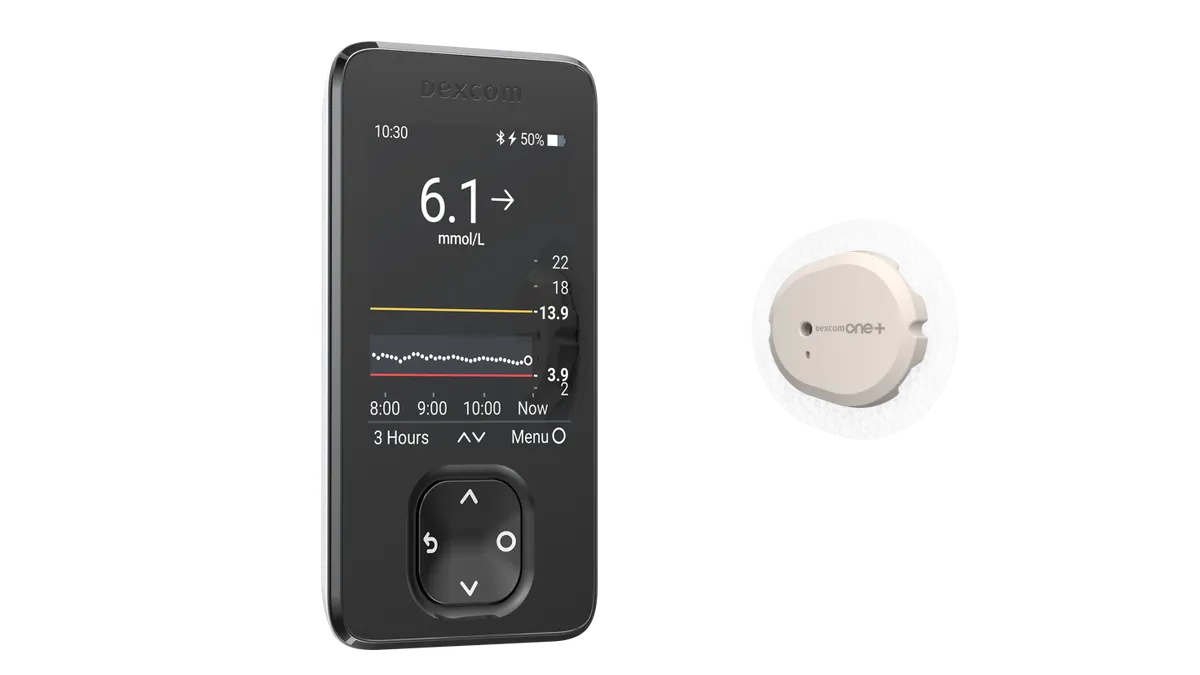
Dexcom recalls more than 700,000 CGM receivers for lack of audible alarm
Severe adverse events, including seizure and loss of consciousness, were potentially linked to the issue, based on 56 reports Dexcom received.
By Elise Reuter • Updated June 24, 2025 -
Zyno recalls infusion pumps over unvalidated software
Customers have been asked to stop using the pumps and wait for someone to contact them about exchanging devices.
By Nick Paul Taylor • June 17, 2025 -
Centerline recalls guidewire over risk of coating being left in patients
Coating for the guidewires could come off and get left in patients, which could result in extended or additional procedures. No serious injuries or deaths have been tied to the recall.
By Nick Paul Taylor • June 16, 2025 -
Medtronic recalls ventilators linked to 2 serious injuries, 1 death
The recall comes more than one year after Medtronic announced it would exit the ventilator market.
By Nick Paul Taylor • June 12, 2025 -
Baxter recalls Novum pump linked to 1 serious injury
The company has asked users to monitor patients frequently to ensure the appropriate infusion is being delivered.
By Nick Paul Taylor • Updated June 11, 2025 -
Medtronic removes tracheostomy tubes due to dislodging risk
If the tube moves out of place, it could prevent a patient from breathing or block the airway. A Medtronic spokesperson said patient harm was reported in some cases.
By Elise Reuter • June 6, 2025 -
Smiths Medical recalls infusion pumps over 3 problems
Smiths has not reported any serious injuries or deaths related to the issues, but the FDA sees a risk of harm.
By Nick Paul Taylor • June 4, 2025 -
BD recalls esophagogastric tubes linked to 2 serious injuries, 1 death
A problem when preparing the devices for use can delay diagnosis or treatment, leading BD to update its instructions.
By Nick Paul Taylor • May 27, 2025 -
HHS overhaul
RFK’s FDA layoffs could slow safety communications, experts warn
The mass cuts, which included communications staff, could slow public notices on medical device recalls and other safety alerts.
By Elise Reuter • May 6, 2025 -
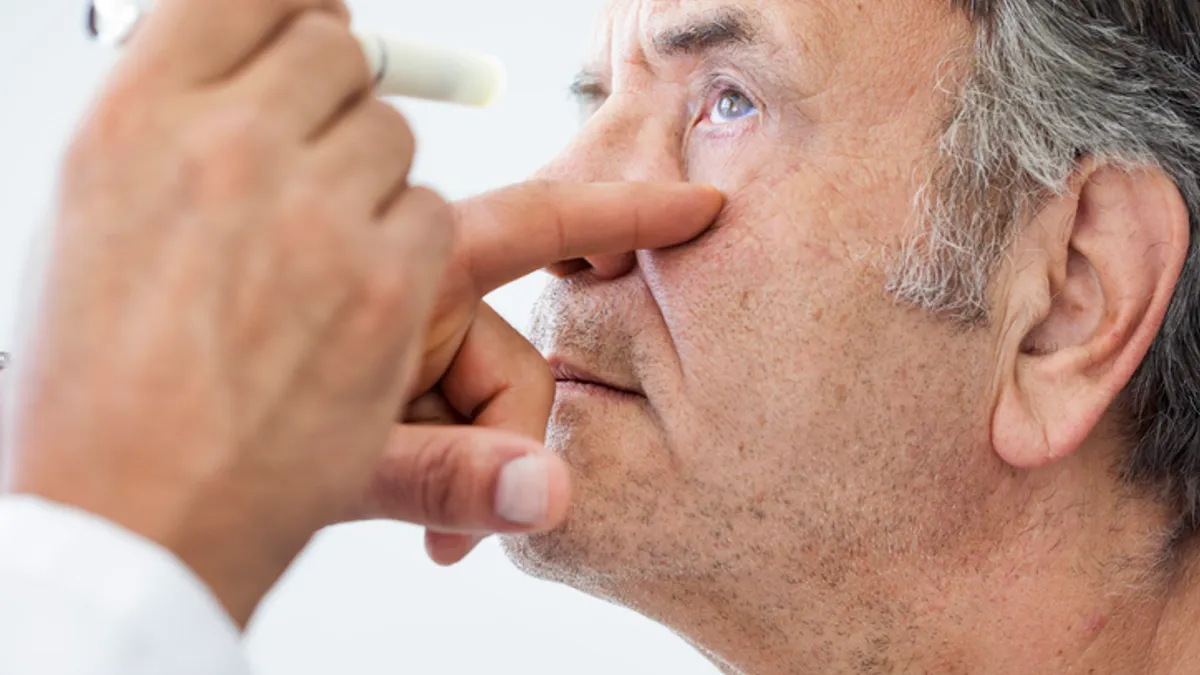
Bausch + Lomb will resume intraocular lens sales weeks after recall
An analysis of the timing of adverse event reports has convinced the company that raw material delivered by a vendor was the source of the problem.
By Nick Paul Taylor • April 28, 2025 -
FDA issues early alert for BD catheters due to leaks
BD recalled certain intravascular catheters tied to reports of 10 serious injuries after finding an increase in cracks from “material fatigue.”
By Susan Kelly • April 21, 2025 -
Bausch + Lomb recalls Envista intraocular lenses over safety risks
J.P. Morgan analysts said it was too early to discuss the financial impact of the recall, but estimated that $70 million to $90 million of revenue could be at risk this year.
By Nick Paul Taylor • March 31, 2025 -

 Retrieved from ICU Medical on March 21, 2025
Retrieved from ICU Medical on March 21, 2025
Smiths Medical recalls port implants, warns on endotracheal tubes
The business, acquired by ICU Medical in 2022, has been dealing with a series of recalls and quality issues over the past several years.
By Susan Kelly • March 21, 2025 -
FDA posts early alert after Calyxo urinary stone device tied to death
The alert describes a problem that can lead to excessive pressure in the kidney during procedures to remove urinary stones.
By Nick Paul Taylor • March 21, 2025 -
Medtronic recalls embolization devices tied to 17 injuries, 4 deaths
Medtronic will remove one model of Pipeline Vantage devices from where they’re used and sold and will update the instructions for another model.
By Nick Paul Taylor • March 19, 2025 -
Top device firms report safety data late: BMJ
BD, Medtronic and Abbott were among the top 10 device companies with the highest number of late reports to the FDA, the study found.
By Elise Reuter • March 18, 2025 -
Philips pulls endovascular implant from market after 20 injuries
Philips stopped selling its Tack endovascular system because of cases where additional interventions were needed to remove the implant.
By Elise Reuter • March 5, 2025 -
Olympus recalls lung biopsy devices linked to 26 serious injuries
The FDA, which published a Class I recall notice, said the tip of the guide sheath has detached from some devices and fallen off in the patient.
By Nick Paul Taylor • March 4, 2025 -
Medtronic recall of brain fluid drainage systems linked to 15 injuries
The company will keep the systems on the market but is asking providers to check devices for visible cracks and return affected devices.
By Nick Paul Taylor • Feb. 4, 2025 -

 Retrieved from Hologic on January 16, 2025
Retrieved from Hologic on January 16, 2025
Hologic receives FDA warning letter over Biozorb implantable markers
Hologic stopped manufacturing the device after reports of serious adverse events in patients who had the markers implanted in breast tissue.
By Elise Reuter • Updated Jan. 16, 2025 -
Philips recall of heart monitor software tied to 109 injuries, 2 deaths
Some electrocardiogram events, such as atrial fibrillation or abnormally rapid heartbeats, over two years were not reviewed by healthcare professionals due to a data routing issue.
By Ricky Zipp • Jan. 14, 2025