Page 164
-
Medtronic recall of capsule delivery devices tied to 33 serious injuries
The Food and Drug Administration published an early alert for the recall on Tuesday. No deaths have been associated with the problem.
-
ADA takeaways: Diabetes tech firms preview new patch pumps, glucose sensors
Medtronic, Beta Bionics and Tandem Diabetes Care showcased planned patch pumps at the conference, while Senseonics shared updates on its implantable glucose sensors.
-
FDA stops Olympus devices from entering the US
The agency said it issued the import alerts because it “continues to have concerns related to outstanding Quality System regulation violations by Olympus.”
-
Moon Surgical hires 3 executives for soft tissue robot expansion push
The challenger to Intuitive Surgical said its Maestro platform has aided in more than 1,600 surgeries to date, with the majority performed in the past year.
-
RFK Jr. details some workers reinstated at HHS
The department reinstated more than 700 workers at the CDC and over 200 employees at the NIH after massive layoffs this spring, HHS Secretary Robert F. Kennedy Jr. told lawmakers on Tuesday.
-
Cassidy challenges RFK Jr. with call for delay to CDC vaccine meeting
The GOP senator objected to Secretary Kennedy's recasting of the influential advisory panel, which has sparked criticism from medical groups and Democratic lawmakers.
-
EU limits Chinese companies’ access to public medtech contracts
The move to block Chinese medical device companies from bidding on EU government contracts is intended to defend EU-based businesses from unfair treatment in China, the European Commission said.
-
Illumina to buy SomaLogic for up to $425M
Illumina said adding SomaLogic would support the company’s multiomics strategy and strengthen the value of its NovaSeq X products.
-
GE Healthcare recalls Carestation devices over ventilation failure risk
The recall affects 15 Carestation models and more than 14,000 individual devices.
-
Insulet’s Eric Benjamin shares update on automated insulin delivery system
The chief product and customer experience officer said Insulet has seen an uptick in users with Type 2 diabetes since receiving an expanded indication, and shared an update on plans for a closed-loop system.
-
MedTech Europe urges EU to exclude devices, diagnostics from trade war
MedTech Europe said the EU’s list of products it could target in retaliation for U.S. actions “includes over 800 trade codes related to medical technologies.”
-
Virtual Incision appoints former Intuitive exec as CEO
Virtual Incision has selected Jim Alecxih as its new CEO as the company prepares a series of FDA submissions for its next robotic surgery system, called M2.
-
5 things to watch at the ADA’s Scientific Sessions
The American Diabetes Association’s annual conference kicks off amid a dynamic year for diabetes tech, as access improves for people with Type 2 diabetes and companies debut new devices.
-
Rise in VC activity tees up ‘strong year’ for medtech funding: PitchBook
Improvements in VC funding contrasted with M&A activity, which has fallen short of the level PitchBook predicted under the Trump administration.
-
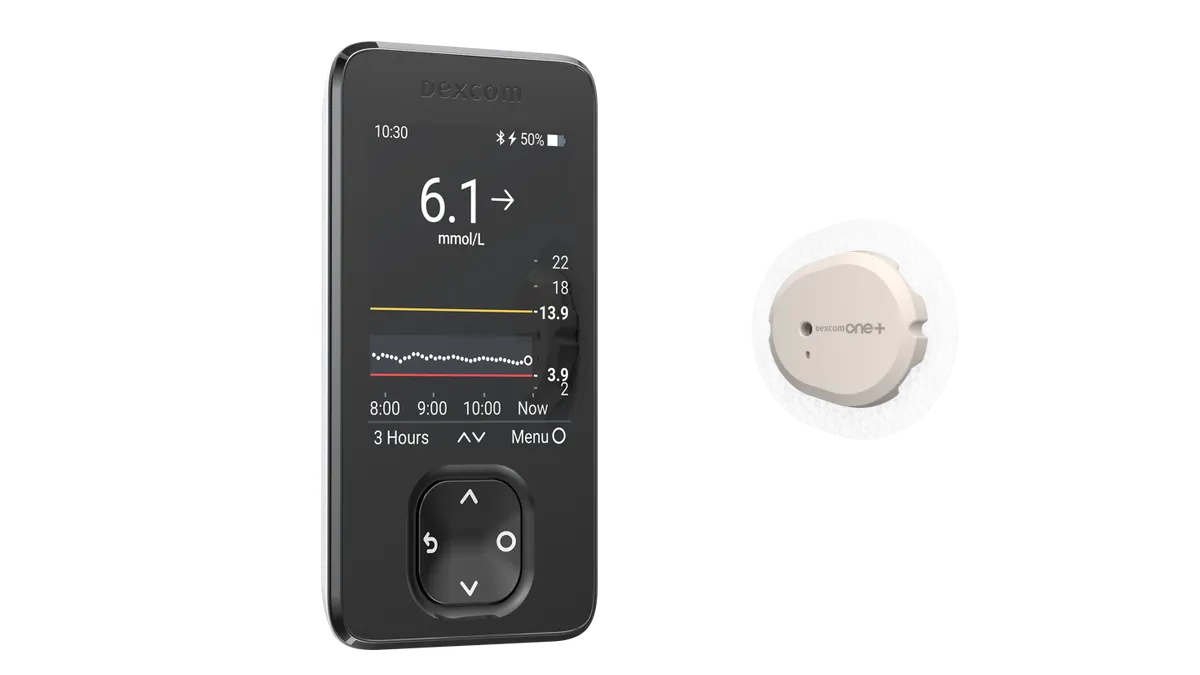
Dexcom recalls more than 700,000 CGM receivers for lack of audible alarm
Severe adverse events, including seizure and loss of consciousness, were potentially linked to the issue, based on 56 reports Dexcom received.
Updated June 24, 2025 -
Medtronic training pact includes Hugo robot, cardiac ablation tech
The medical device maker is partnering with IRCAD, a surgical education and research institute, to train medical personnel in cardiovascular, neuroscience and minimally invasive specialties.
-
Q’Apel recalls clot removal device in response to FDA warning letter
Rather than pursuing a new regulatory pathway, Q'Apel said it is discontinuing the recalled system “as part of its strategic shift toward newer technologies.”