Page 2
-
Genetic testing takes greater share of Medicare Part B test spending: OIG
Spending on the tests, which analyze genetic material from both people and pathogens, rose 20% year over year in 2024, the analysis found.
-
Abbott receives FDA warning letter over Freestyle Libre CGMs
Abbott needs to conduct more performance testing to ensure its FreeStyle Libre devices are accurate, FDA inspectors found.
-
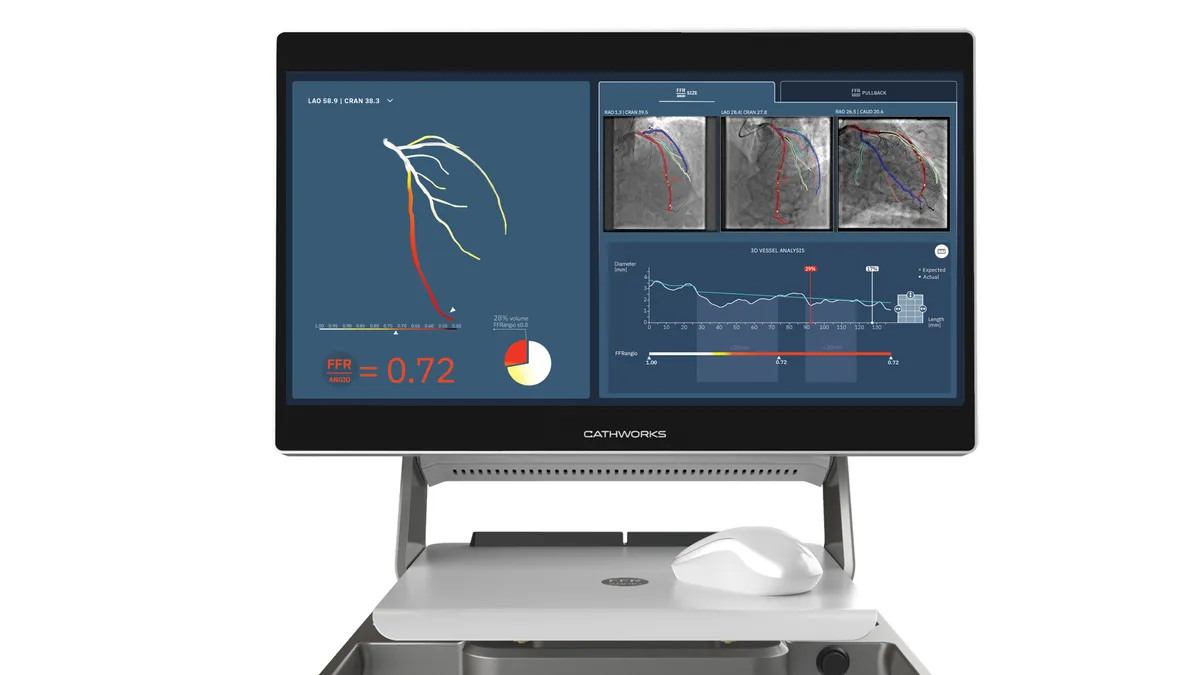
Medtronic to acquire CathWorks for up to $585M
The purchase will give Medtronic a larger presence in the cath lab, with a tool to help diagnose and treat coronary artery disease.
-
Beta Bionics receives FDA warning letter
The letter, which the FDA has not yet published, does not affect Beta Bionics’ ability to market, manufacture or distribute products, the company said.
-
STAAR names interim co-CEOs amid search for permanent leader
The company is looking for a new top executive after former CEO Stephen Farrell left when STAAR shareholders rejected a buyout offer from Alcon.
-
Lawsuit challenges ethylene oxide exemptions for device sterilizers
The lawsuit questions the Trump administration’s authority to make sweeping environmental exemptions without evidence.
-
Grail files for FDA approval of multi-cancer early detection test
Premarket approval could help the company secure coverage from commercial payers and Medicare.
-
ACA enrollment backslides to 23M in 2026
That’s down 5% from last year. But it’s not the nosedive some market watchers predicted, and a handful of states — notably, Texas — saw robust growth.
-
Top medtech trends to watch in 2026
From M&A to surgical robotics and user fee negotiations, the medical device industry has a busy year ahead. Check out MedTech Dive’s roundup of the top medtech trends to watch in 2026.
-
Stryker starts first cases with handheld ortho robot
Stryker expects the robot, called Mako RPS, to bring in new customers, including more ambulatory surgery centers.
-
Senseonics wins CE mark for one-year CGM
The company is hiring sales reps and transitioning staff from its former partner to support launches in Germany, Italy, Spain and Sweden.
-
Deep Dive
5 robotic surgery trends to watch in 2026
Soft tissue robotic systems designed to improve surgical precision are expanding into new specialties and increasing competition for longtime industry leader Intuitive Surgical.
-
CDRH on track for review timelines despite staff cuts
The Center for Devices and Radiological Health said it is on track to meet review times specified by user fee agreements, despite “resource challenges.”
-
Top medtech conferences in 2026
The medical device industry has a jam-packed year of events for professionals to attend, including the J.P. Morgan Healthcare Conference, DeviceTalks Boston and AdvaMed’s annual medtech meeting.
Updated Jan. 28, 2026 -
Cleveland Diagnostics names Michael Iskra CEO
Iskra will help Cleveland Diagnostics scale up commercial adoption of its IsoPSA blood-based test that analyzes the structure of protein biomarkers for prostate cancer signals.
-
Medtech M&A took off in the second half of 2025: report
Acquisitions and spinoffs in 2025 surpassed the previous three years, according to a report from Bain & Company.
-
Edwards’ request for broader TAVR Medicare coverage gets pushback
Several medical societies supported extending Medicare coverage for TAVR to patients with asymptomatic aortic stenosis, but some physicians and rival Medtronic said more data is needed.
-
BD, Waters set completion date for $17.5B biosciences and diagnostics merger
With Waters stockholders voting to issue shares, the companies are targeting a Feb. 9 close date for the deal.
-
FDA guidance eases wearables oversight. But experts have questions about what’s next.
Experts say two new guidances — issued without the usual public comment period — leave questions about how patients will navigate a growing pool of wearables.
-
Medtronic targets ASCs with extended Mindray collaboration
Mindray is integrating Medtronic patient monitoring technologies into its platforms to meet the needs of ambulatory surgery centers.
-
Intuitive’s cardiac initiative includes valve repair, LAA closure
With nine new procedures cleared for the da Vinci 5 robot, Intuitive’s strategy includes working with a limited number of U.S. sites this year to establish cardiac programs.
-
Medtronic wins CE mark for single-shot pulsed field ablation catheter
Medtronic’s Affera Sphere-360 is a single-shot device, putting it in direct competition with Boston Scientific’s Farawave catheter.
-
Intuitive pushes into cardiac robotic surgery, ASCs
The robotics giant revealed on an earnings call that it won FDA clearance for several cardiac procedures and is also ramping up its presence in ambulatory surgery centers.
-
Congress eyes tackling healthcare consolidation
“I think there’s common ground here,” one Republican congressman said Wednesday during a House Budget Committee hearing. Squaring up against healthcare monopolies would be a major pivot for the GOP.
-
AdvaMed leaders on tariffs, MDUFA and wearables
AdvaMed CEO Scott Whitaker and Mick Farrell, new board chair of the trade group, weighed in on important issues for the medtech industry one year into the Trump administration.