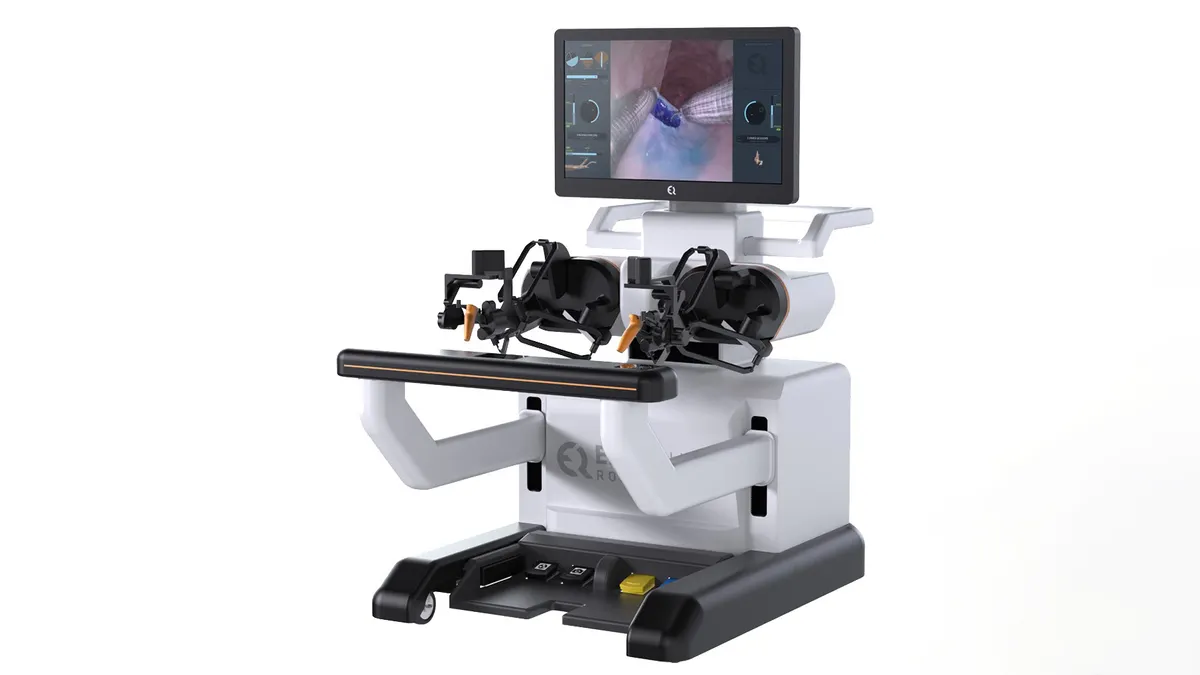
EndoQuest Robotics, developer of an endoluminal robotic surgery system, said Thursday that the first procedures in its pivotal trial were completed at HCA Houston Healthcare Medical Center.
The technology is designed to navigate through natural body openings. EndoQuest received an investigational device exemption from the Food and Drug Administration in December to study its system in procedures to remove colorectal lesions. The clinical trial’s first two endoscopic submucosal dissection procedures were performed on complex lesions in the rectum and sigmoid colon.
The surgery uses an endoscope to remove lesions under the lining, or mucosa, of the gastrointestinal tract. The minimally invasive procedure has not been widely adopted in the U.S. due to its technical difficulty. EndoQuest’s robotic system is intended to address challenges with the surgery, allowing more physicians to adopt the technique in place of more invasive procedures such as colectomies, Chief Medical Officer Todd Wilson said in a statement.
Houston-based EndoQuest’s Paradigm study will enroll 50 patients at five U.S. centers: Brigham and Women’s Hospital in Boston; Mayo Clinic in Scottsdale, Arizona; the Cleveland Clinic; AdventHealth in Orlando, Florida; and HCA Houston.
EndoQuest plans to submit a de novo request for authorization to market the system in the U.S. once the trial is completed, the company said.
Virtuoso performs first cases
Virtuoso Surgical’s robotic endoscopy system was used to successfully perform bladder lesion excision in six patients during the pilot phase of the Viable trial at The Chinese University of Hong Kong, CEO Duke Herrell posted last week on LinkedIn.
Virtuoso is working to improve a surgical technique for lesion removal in suspected bladder cancer using its robotic technology, Herrell said.
The robotic system has two needle-sized manipulators that work from the tip of an endoscope, enabling surgeons to use two hands to perform maneuvers deep within the body.
The Nashville, Tennessee-based medical device company plans to submit an IDE application to the FDA to study the system’s use in the U.S.