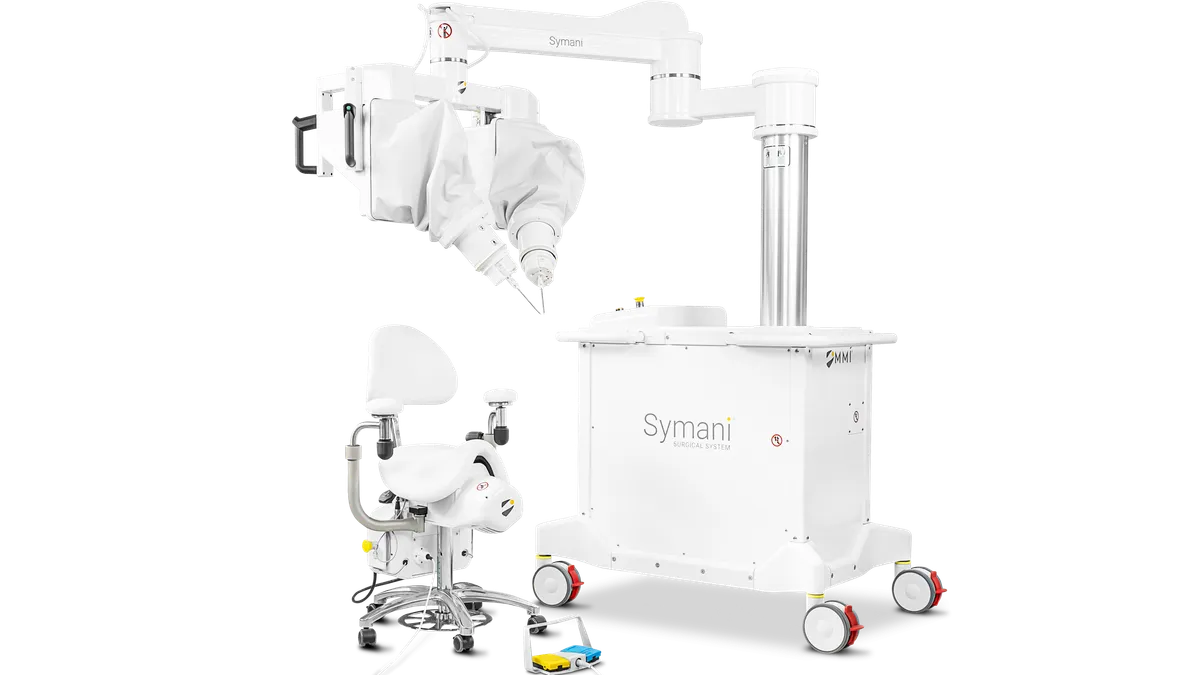
MMI said the Food and Drug Administration approved an investigational device exemption to study a microsurgical intervention for Alzheimer’s disease using the company’s Symani robotic platform.
The REMIND study will evaluate the safety and feasibility of the procedure to improve drainage of neurotoxins, such as amyloid beta and phosphorylated tau, from the brain in patients with mild to moderate Alzheimer's disease and obstruction in the deep cervical lymph nodes of the neck.
The study’s primary endpoint is device-related serious adverse events through 30 days after the procedure. Additional endpoints include biomarker and imaging changes, and cognitive assessments through six months.
MMI said few surgeons are able to dissect and suture delicate lymphatic vessels without robotic assistance, noting the structures can be 0.2 millimeters in diameter, equal to the thickness of two sheets of paper.
The Symani system combines microinstruments with tremor-reducing technology. It won FDA de novo authorization last year for microsurgery. Last month, MMI said it completed the first cases in a feasibility trial assessing the system for restoring blood supply to the brain in people with Moyamoya disease, a condition where the carotid artery in the skull becomes blocked or narrowed.
The company said last week it is partnering with research institutions in the U.S. and Europe to begin patient enrollment in the Alzheimer’s study.
SS Innovations’ new console used in telesurgery
SS Innovations International said the first telesurgery was performed with a compact version of its SSi Mantra surgeon command center. CEO Sudhir Srivastava, a heart surgeon, performed the coronary artery bypass surgery remotely on Oct. 17.
From his New Delhi residence, Srivastava operated on a patient about 185 miles away, at Manipal Hospital in Jaipur, India, the company said Thursday.
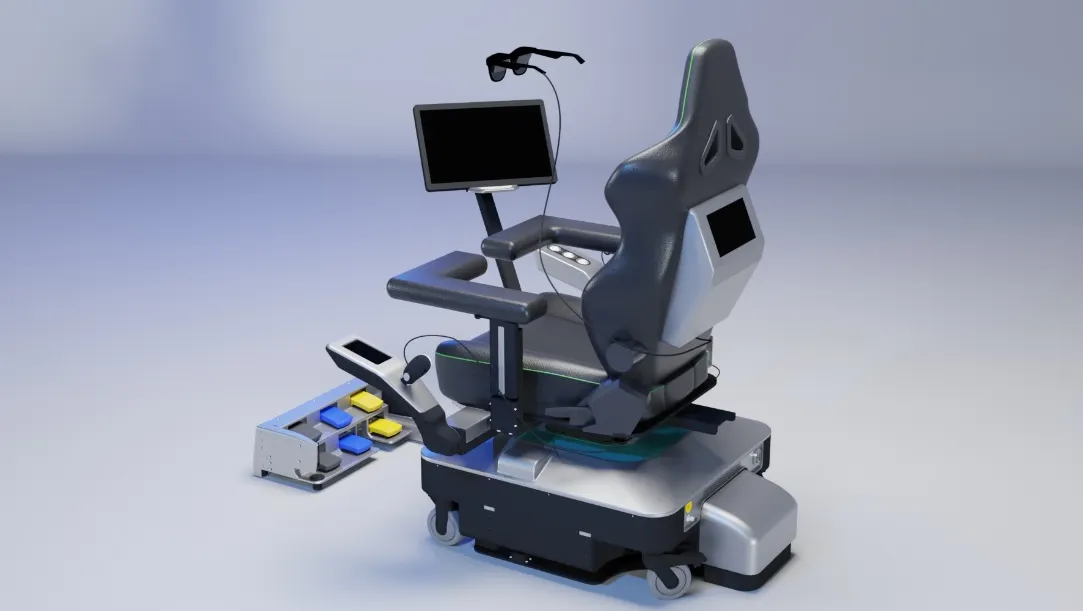
SS Innovations’ SSi Mantra soft tissue robot has been used in telesurgeries before, including for some heart procedures. The new SSi Mantra Tele Surgeon Console requires less space, which should enable telesurgery from more locations, including physicians’ offices, the company said.
SS Innovations expects use of the smaller console, which has built-in electronics and magnified three-dimensional view capabilities, will help increase patient access to surgical expertise.
In the U.S., SS Innovations plans to submit a 510(k) application for the SSi Mantra platform to the FDA in the fourth quarter. Outside the U.S., in countries where the system is already approved, more than 6,500 surgeries have been completed with the robot.
Procept CEO: Hospitals watching capital spending
Procept BioRobotics said it is focused on driving utilization of its aquablation systems in an uncertain economic environment, after posting a 43% increase in third-quarter revenue.
Larry Wood, in his first earnings call as CEO, said last week that Procept has begun to see some large U.S. hospital systems scrutinize capital spending more carefully due to “evolving” macroeconomic conditions. Procept is focused on improving procedure utilization with its systems and expanding in international markets.
To support the initiatives, the company has hired Pooja Sharma Rao from Edwards Lifesciences, where she led marketing and strategy, Wood said.
Wood joined Procept in September after 40 years with Edwards, where he managed the transcatheter heart valve business.
Procept, whose aquablation procedure uses pressurized fluid to cut tissue to treat enlarged prostate, said quarterly revenue rose to $83.3 million on higher shipments of its robotic systems to new hospital customers. The U.S. installed base grew by 58 systems in the quarter to 653 in total.
The company reported a net loss of $21.4 million in the third quarter, compared to a $21 million loss in the year-ago quarter. Procept projected full-year 2026 revenue in a range of $410 million to $430 million, up 26% to 32% from its expected 2025 revenue.
The company’s next-generation Hydros robotic system received FDA clearance in August 2024.