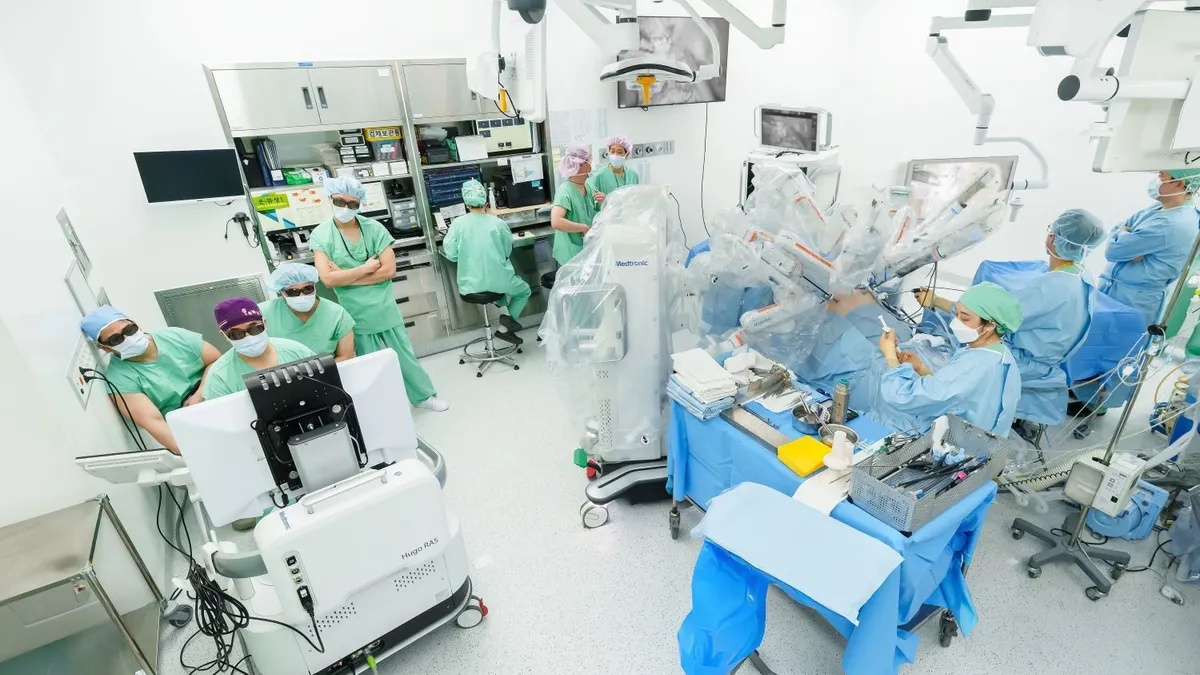
Dive Brief:
- Medtronic said Wednesday it obtained U.S. clearance for its Hugo system in urologic procedures, positioning the medtech giant to challenge Intuitive Surgical in the world’s largest robotic surgery market.
- In addition to the initial urology indication, which includes common procedures such as prostate removal, the company plans to extend use of the robot to more surgical specialties in the U.S. over time, with general surgery and gynecology expected to follow next.
- The Food and Drug Administration clearance “means there is now choice” for hospitals looking to expand their robotic programs, Rajit Kamal, Medtronic’s vice president and general manager of robotic surgical technologies, said in a statement.
Dive Insight:
With the FDA’s authorization, Medtronic becomes the first large medtech company to introduce a robotic system for soft tissue surgery in the U.S. since Intuitive’s arrival more than two decades ago. Johnson & Johnson is looking to enter the market with its Ottava robot and expects to make an FDA submission in early 2026 after encountering delays. Dozens of smaller companies are also working to launch surgical robots they hope will contend for a piece of the market.
Medtronic received Europe’s CE mark for Hugo in 2021. First unveiled as a challenger to Intuitive’s da Vinci system in 2019, the robot is now available in more than 30 countries worldwide and has been used in tens of thousands of urologic, gynecologic and general surgery procedures to date, according to the company.
While Medtronic has not disclosed the size of its global installed base, it has indicated procedure growth with the robot remains in the double digits, BTIG analysts wrote Wednesday.
“As [Medtronic] picks up additional clearances, we think momentum can begin to build,” the analysts said.
Medtronic is a leader in the surgical instruments market. Given its size and scale, “we would not be surprised” to see the company bundle Hugo with other products it sells to health systems “to place more systems and drive utilization of instrumentation,” the analysts wrote.
CEO Geoff Martha, on an earnings call last month, said Hugo’s expansion is expected to help revive growth in Medtronic’s medical/surgical portfolio, which has faced pressure from the market’s shift to robotics. The company sees the platform’s modular design, open surgeon console and digital technologies as differentiating features.
Medtronic did not provide details on the timing of its U.S. launch. Stifel analysts predicted a “slower, more deliberate approach” to the rollout initially, with momentum expected to accelerate once the system gains more labeling indications.
In October, Medtronic said its U.S. clinical trial studying Hugo in gynecological procedures had begun, and the company announced positive results from the robot’s pivotal hernia repair study in September.