Dive Brief:
- Medtronic has received Food and Drug Administration clearance for a next-generation spine surgery system that combines artificial intelligence-based planning, real-time navigation and robotic assistance.
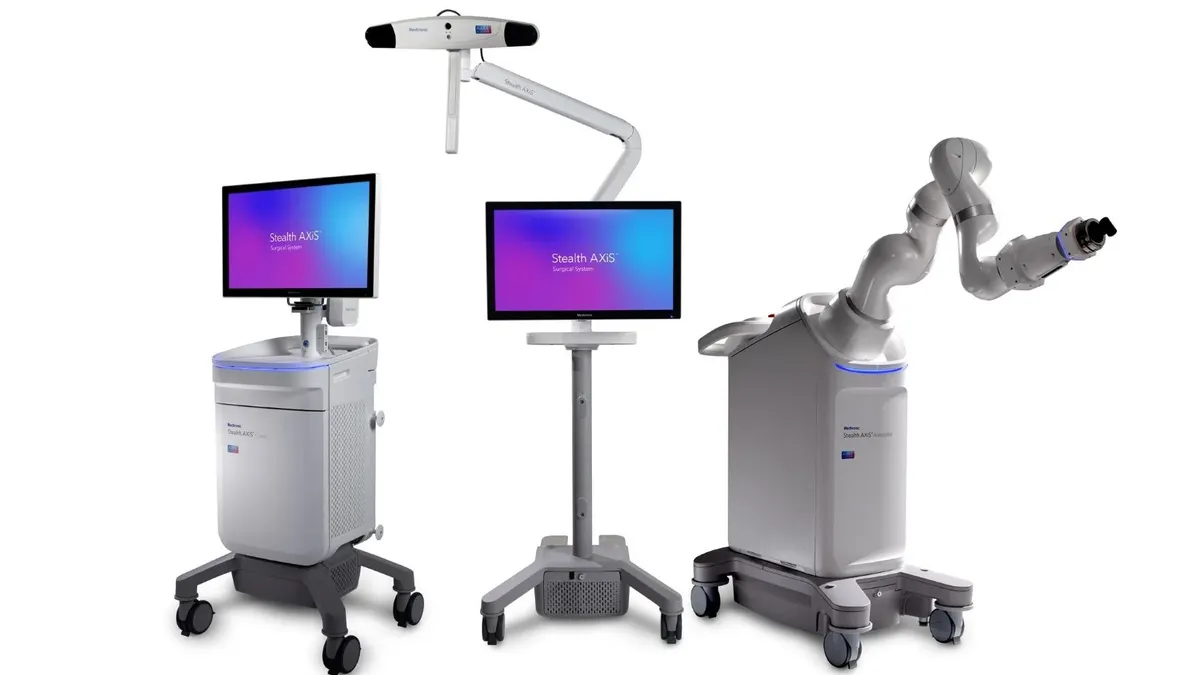
- The Stealth AXiS system has a modular design that can be used in both hospital settings and ambulatory surgery centers and can accommodate a range of surgeon preferences, Medtronic said Friday.
- The underlying architecture can also support cranial applications and ear, nose and throat surgeries, pending 510(k) clearance, according to the device maker.
Dive Insight:
Medtronic, a leader in spinal technologies, added robot-assisted surgical guidance to its portfolio with the $1.64 billion acquisition of Mazor Robotics in 2018.
The Stealth AXiS system builds on Mazor’s robotics capabilities and Medtronic’s StealthStation navigation technology, according to the company. The system also connects with Medtronic’s AiBLE ecosystem combining AI, data and services to support personalized planning, surgical execution and post-operative analysis.
A key advance is the ability for surgeons to visualize anatomic motion, surgical adjustments and patient alignment in real time during spine surgery, without the need for repeated imaging, Medtronic said.
Medtronic’s competitors in the spine market include Johnson & Johnson and Globus Medical. J&J, however, plans to spin out its DePuy Synthes orthopedics business, which includes a spine robotics and navigation platform. The company said in October that it expects the separation to happen in the next 18 to 24 months.
Medtronic, meanwhile, told investors at the J.P. Morgan Healthcare Conference this year that it is gaining market share in the $15 billion cranial and spinal technologies market, and that it views the sector as an attractive segment that is at a “technology inflection point.” Medtronic said the AiBLE ecosystem has an installed base that is 10 times larger than the nearest competitor.
Medtronic also has a partnership with Siemens Healthineers to integrate the AiBLE suite of spine surgery products with Siemens Healthineers’ Multitom Rax robotic X-ray system for pre- and post-operative imaging.
Stryker, another big orthopedics player, last year sold its U.S. spinal implants business to Viscogliosi Brothers, which created VB Spine. Stryker said it remained committed to the spine market through its interventional spine, neurotechnology and enabling technologies businesses and a strategic partnership with VB Spine. In May, Stryker obtained Food and Drug Administration clearance for its OptaBlate device to treat lower back pain.