Dive Brief:
- Medtronic said Tuesday it received the CE mark in Europe for its LigaSure vessel-sealing technology for use in robotic-assisted surgeries.
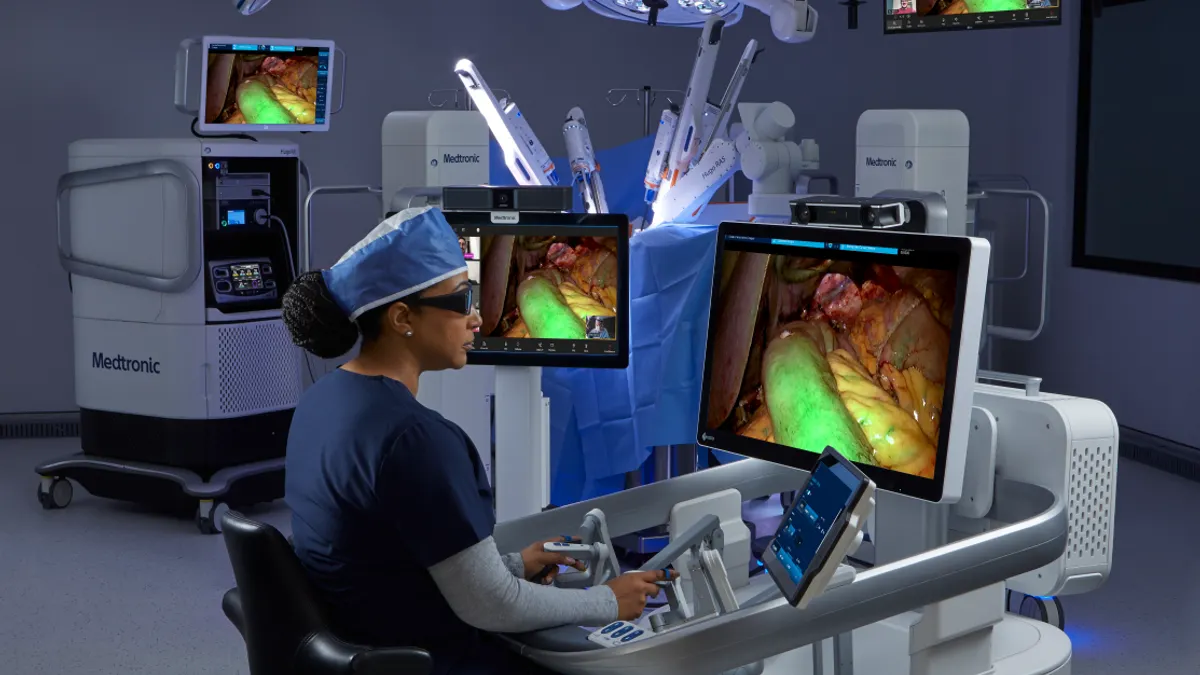
- The designation increases the capabilities of Medtronic’s Hugo soft tissue robotic system for gynecologic, general and urologic procedures in Europe.
- Hugo is not yet for sale in the U.S. Medtronic said it expects Hugo to enter the U.S. market later in the company’s current fiscal year, which ends in April 2026, with an initial indication in urology.
Dive Insight:
Medtronic is continuing its push into the fast-growing robotic surgery market led by Intuitive Surgical.
Expansion of the Hugo platform is key to the company’s strategy for improving growth in its surgical business, and the market-leading LigaSure vessel sealing technology is attracting strong surgeon adoption and taking share in the advanced energy field, CEO Geoff Martha told investors on the company’s fourth-quarter earnings call in May.
In its CE mark announcement at the Society of Robotic Surgeons Annual Meeting in Strasbourg, France, Medtronic said the LigaSure device reliably seals vessels in about two seconds and minimizes the spread of thermal energy to the surrounding tissue.
“LigaSure technology is one of the most important advances for minimally invasive surgery because it provides sealing and cutting in a way that is very, very secure for the performance of the surgeon and the security of the patient,” Miguel Caceres, of the Pacifica Salud Hospital in Panama, said in Medtronic’s statement. “As a robotic surgeon, we want LigaSure technology because it gives us confidence in the security of the seal.”
Hugo first gained the CE mark in Europe in 2021 and is now in use in more than 30 countries. In the U.S., the medical device giant submitted its application to the Food and Drug Administration for a urology indication for Hugo in the first quarter of this calendar year. Expansions into hernia and gynecology are planned.
Medtronic said it also planned a live telesurgery demonstration with Hugo and would share new gynecologic data for the system at the Strasbourg meeting.