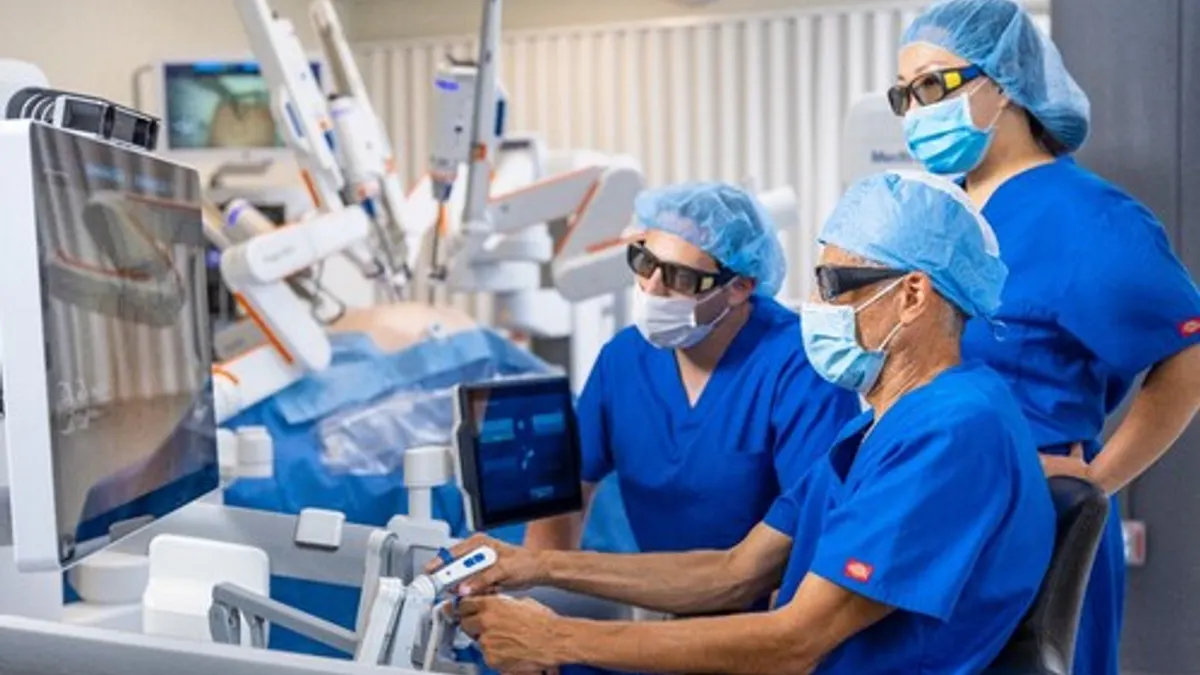
Dive Brief:
- Medtronic said Wednesday it has completed the first procedures in a U.S. clinical study assessing the safety and effectiveness of its Hugo robot in gynecological procedures.
- Gynecology is the focus of Medtronic’s third investigational device exemption study in the U.S., after trials for urology and hernia repair met their primary safety and effectiveness endpoints.
- The total hysterectomy procedures were performed at AHN West Penn Hospital in Pittsburgh. Medtronic expects to enroll as many as 70 people across up to five U.S. hospitals, and include patients having radical, modified radical or total hysterectomies, as well as those being treated for malignancies.
Dive Insight:
Medtronic is gearing up to challenge robotic surgery market leader Intuitive Surgical in the U.S. The Food and Drug Administration is now reviewing the company's first application for Hugo, submitted in the first quarter, for a urology indication.
Medtronic has said it expects to enter the U.S. market later in its current fiscal year, which ends in April 2026, with an initial indication in urology, followed by expansions into hernia and gynecology.
In trial results shared last month for hernia repair, the company said the data showed a 100% surgical success rate, exceeding the 85% performance goal, in a study of 193 people.
Medtronic marked another milestone in its robot ramp-up in September when it announced it would double the size of its London hub for AI and robotics. The Hugo soft tissue robot is already available in more than 30 countries, including in Europe. In the U.S., Medtronic is collaborating with the non-profit IRCAD North America on surgical education initiatives, including for Hugo.
The medtech giant sees robot-assisted procedures as enabling fewer complications and shorter hospital stays for patients than traditional open surgery.
The U.S., where Intuitive is rolling out its latest-generation da Vinci 5 system, will be an important battleground in Medtronic’s Hugo expansion efforts. Medtronic CEO Geoff Martha has said the robot is expected to support a rebound in growth in the company’s surgical business.