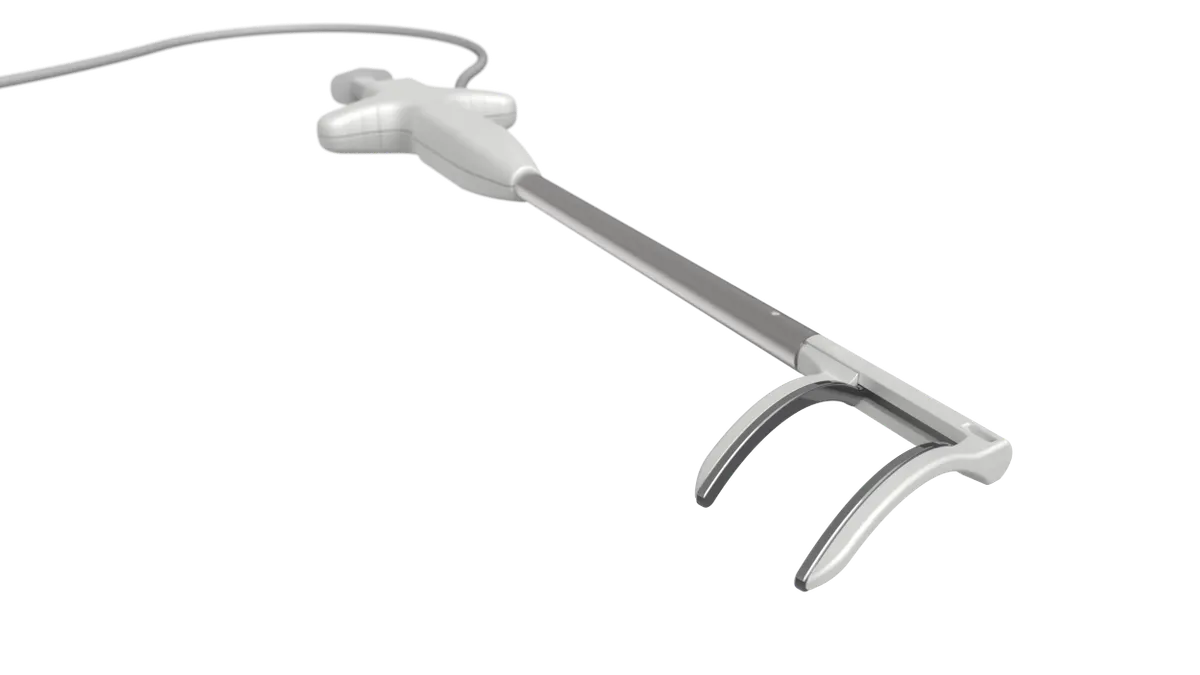
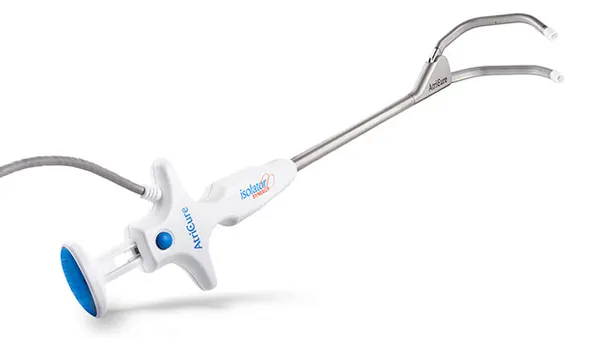
Pulse Biosciences said Monday it received Food and Drug Administration approval to begin a clinical study of its pulsed field ablation system to treat atrial fibrillation during concomitant cardiac surgeries.
The investigational device exemption clears the way for Pulse to start the NANOCLAMP AF study for its nsPFA cardiac surgical device. The single-arm prospective study, designed to demonstrate the system’s primary effectiveness, is expected to enroll as many as 136 patients at up to 20 sites, including two outside the U.S.
Pulse is the first company to advance PFA into the cardiac surgical field for the treatment of AFib, CEO Paul LaViolette said in a statement.
Niv Ad, Pulse’s chief science officer for cardiac surgery, said the company believes its proprietary nonthermal mechanism of cardiac ablation offers significant safety, effectiveness and speed improvements over current thermal methods, such as radiofrequency ablation.
Galvanize raises $100M, taps Godshall as CEO
Galvanize Therapeutics has raised $100 million in new funding to further develop pulsed electric field treatments for cancer and lung disease, and named Doug Godshall, former CEO of Shockwave Medical and HeartWare International, as chairman and CEO.
The technology uses electrical pulses to target cells involved in disease processes. The funds will support commercial expansion and the continued development of Galvanize’s Aliya platform for solid tumors and RheOx device for chronic bronchitis, the Redwood City, California-based company said last week. The Aliya system is cleared in the U.S. for the ablation of soft tissue, while RheOX is an investigational device.
“There are millions of patients who are underserved by today's largely pharmaceutical approaches, and I feel fortunate to be able to join the team as we seek to meaningfully improve the outcomes of those who are suffering with these chronic diseases," Godshall said in a statement.
The Series C round was led by Sofinnova Partners, with investment from Intuitive Surgical, Norwest Venture Partners, Fidelity Management & Research, T. Rowe Price, Gilmartin Capital and others. Galvanize also secured $100 million in a 2022 Series B round.
Godshall takes the CEO reins from Galvanize founder Jonathan Waldstreicher, who has stepped into the role of president and chief strategy officer.
Godshall was CEO of Shockwave, maker of a treatment for calcified plaque, through its $13.1 billion acquisition by Johnson & Johnson in 2024. Before that, he was CEO of heart pump maker HeartWare International, which Medtronic bought for $1.1 billion in 2016. Medtronic would pull the recall-plagued HeartWare device from the market five years later.
Steven Mickelsen starts another PFA company
PFA pioneer Steven Mickelsen announced the launch of Del Medtech, a seed-stage medical device company focused on ablation technologies for therapeutic areas beyond cardiac care.
Del Medtech is developing single-use ablation devices designed to improve efficiency and patient outcomes in specialties including gastroenterology; ear, nose and throat; men’s and women’s health; and oncology.
"We are entering a period of rapid acceleration in ablative therapies, much like the rise of PFA in electrophysiology," Mickelsen, the inventor of Boston Scientific’s Farapulse PFA system, said in a statement. "Del Medtech is poised to lead this wave, and we welcome investors and partners who want to be part of shaping the future of ablation."
The company, founded in 2024 in Cardiff-by-the-Sea, California, said it is targeting market opportunities with annual revenues ranging from $1.4 billion to $7.4 billion and projected growth rates between 9% and 13%.
In 2022, Mickelsen founded Field Medical to develop a PFA approach for ventricular tachycardia, an irregular heart rhythm.