New blood pressure treatments from Medtronic and Recor Medical will now be covered by Medicare.
The Centers for Medicare and Medicaid Services on Tuesday finalized a national coverage determination for renal denervation to treat people with uncontrolled hypertension, a widespread condition that raises the risk of heart disease and stroke.
The decision is expected to increase use of the technology to fill a treatment gap for patients when lifestyle changes and prescription medications have failed to lower their blood pressure.
Cardiologists told the CMS in public comments that the patients they have treated with a renal denervation procedure have seen significant blood pressure reduction. However, limited insurance reimbursement has slowed clinical adoption of the treatment.
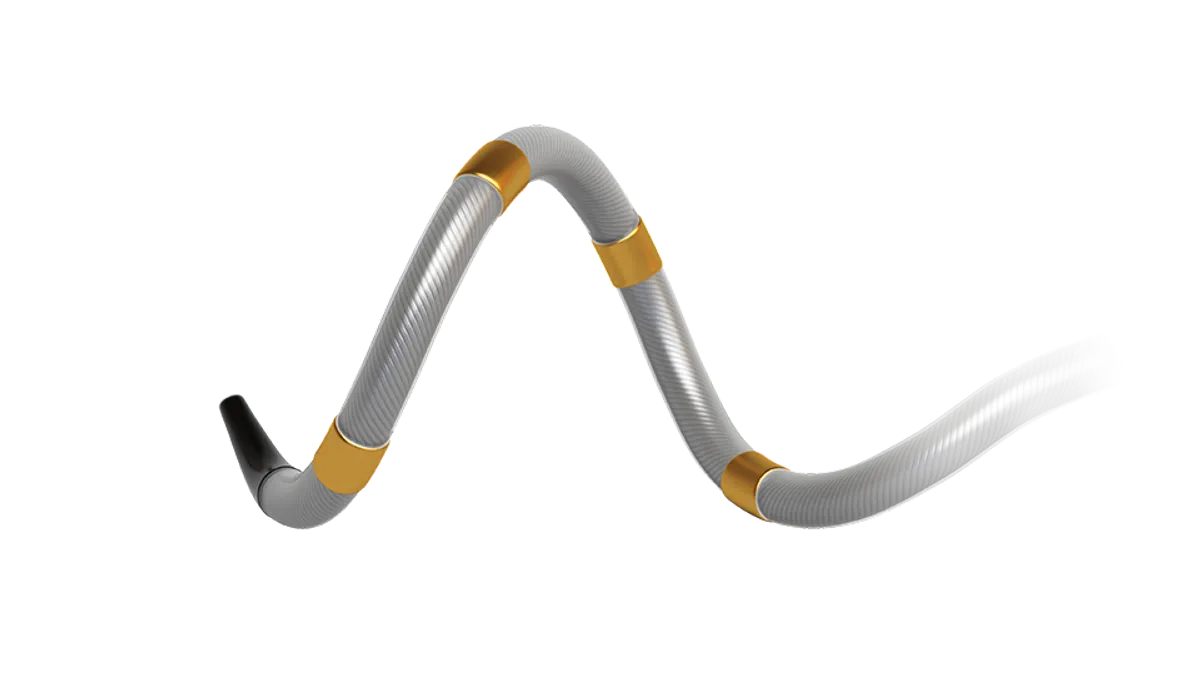
In the catheter-based procedure, ultrasound or radiofrequency energy is delivered to ablate nerves around the renal arteries, disrupting overactive signaling between the kidneys and the brain to reduce blood pressure.
Two renal denervation devices, Medtronic’s Symplicity Spyral and Recor Medical’s Paradise, won Food and Drug Administration approval to treat drug-resistant hypertension in late 2023. The NCD covers both systems.
“By formally recognizing the proven clinical value of renal denervation and extending Medicare coverage, CMS is paving the way for broader, more equitable access to this breakthrough therapy,” Lara Barghout, CEO of Recor, said in a statement after the CMS decision.
Medtronic requested the NCD from the CMS in December 2024, stating that a hypertension epidemic in the U.S. shows there is an unmet need for a new option to help improve blood pressure management. Almost half of U.S. adults have hypertension, and only 1 in 4 have it under control.
Medtronic expects the treatment to be an important new product for the company.
"This determination represents a significant opportunity to improve care for patients and opens a novel and meaningful market for Medtronic, positioning our renal denervation technology as one of the most exciting growth drivers for the company,” said Skip Kiil, president of Medtronic’s cardiovascular portfolio.
Coverage criteria
The NCD recommends renal denervation for patients meeting several criteria, including a diagnosis of uncontrolled high blood pressure, defined as a reading higher than or equal to 140 mm Hg systolic blood pressure and higher than 90 mm Hg diastolic blood pressure.
Patients should be under the care of a clinician with primary responsibility for blood pressure management.
Before getting a referral for renal denervation, patients must be on lifestyle modifications and stable doses of guideline-directed medical therapy for at least six weeks and under the primary clinicians’ care for a minimum of six months, having at least three visits, with no more than two of the visits being virtual.
Years in development
Both Recor and Medtronic worked for more than a decade to bring renal denervation systems to market. Recor, a subsidiary of Otsuka Medical Devices, was founded in 2009 to focus on developing the Paradise ultrasound system.
Medtronic started working on its technology the same year. The investment continued after Medtronic acquired startup Ardian, developer of the Symplicity radiofrequency catheter, in 2011.
Along the way, Medtronic faced some disappointing study results that failed to demonstrate significant blood pressure reduction, leading an FDA advisory panel to vote against recommending the device. But the procedure ultimately won FDA approval, supported by pivotal trial data demonstrating safety and effectiveness.
“Medtronic management has been building out its commercial infrastructure for its Symplicity Spyral RDN system, and we expect physicians to more rapidly adopt the technology now that a NCD is in place,” Citi Research analyst Joanne Wuensch wrote in a research note. “Still, as an emerging therapy, early utilization is likely measured as surgeons collect their own data and determine which patients may receive the most benefit.”