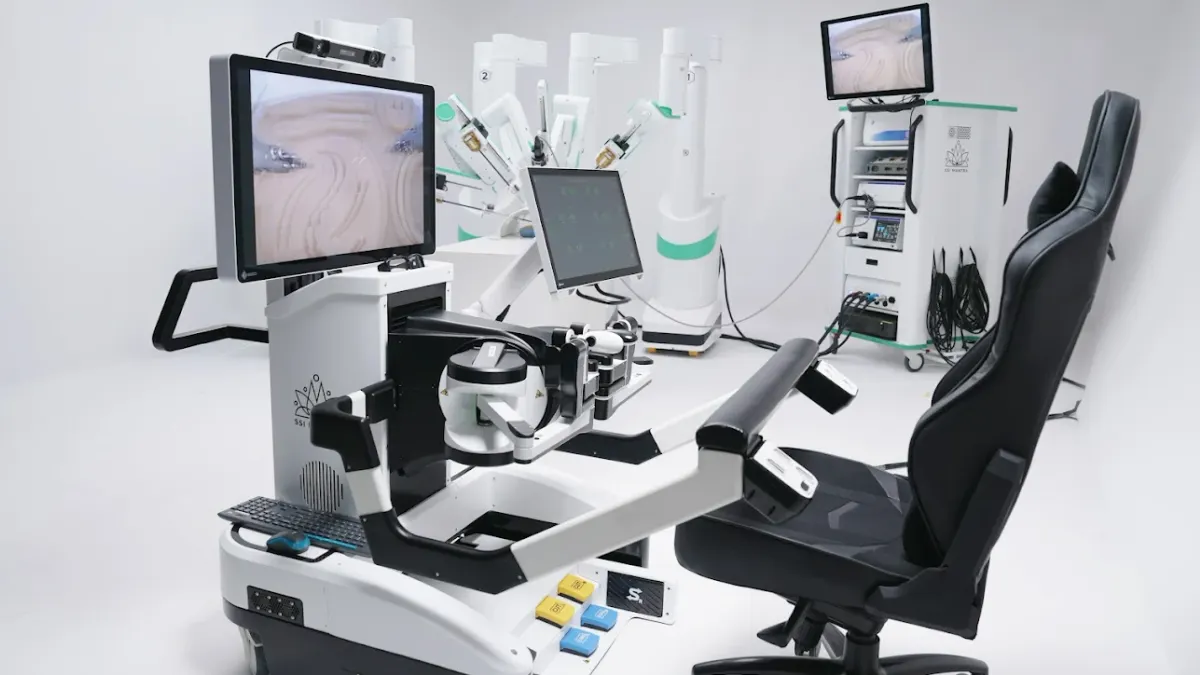
SS Innovations International said Thursday it expects to seek 510(k) clearance for the SSi Mantra surgical robot in the fourth quarter and could get the go-ahead to market the system in the U.S. in the first half of 2026, based on typical review timelines.
The company said it had planned to file a de novo request with the Food and Drug Administration but decided to pursue the 510(k) pathway instead to potentially save time and costs.
In September, SS Innovations completed a human factors validation study for the soft tissue robot at Johns Hopkins Hospital, meeting the FDA’s usability and patient safety requirements. The study will be included as a key part of the 510(k) submission.
The India-based company, which intends to apply for multiple surgical specialty indications for the SSi Mantra system in the U.S., said the projected timeline to receive FDA clearance excludes any time needed for additional information requests.
SS Innovations is gearing up to challenge Intuitive Surgical in the U.S. soft tissue robotic surgery market, and is also pursuing the CE mark in Europe. As of Sept. 30, a total of 125 SSi Mantra systems have been installed in countries where the robot is approved. More than 6,000 surgical procedures have been performed, including 60 telesurgeries and 310 cardiac procedures.
Last month, Naveen Kumar Amar joined the company as CFO to support its global expansion.