Dive Brief:
- Terumo plans to acquire OrganOx, a maker of organ preservation devices, in a roughly $1.5 billion deal that will allow the Tokyo-based medical device maker to enter the transplantation sector.
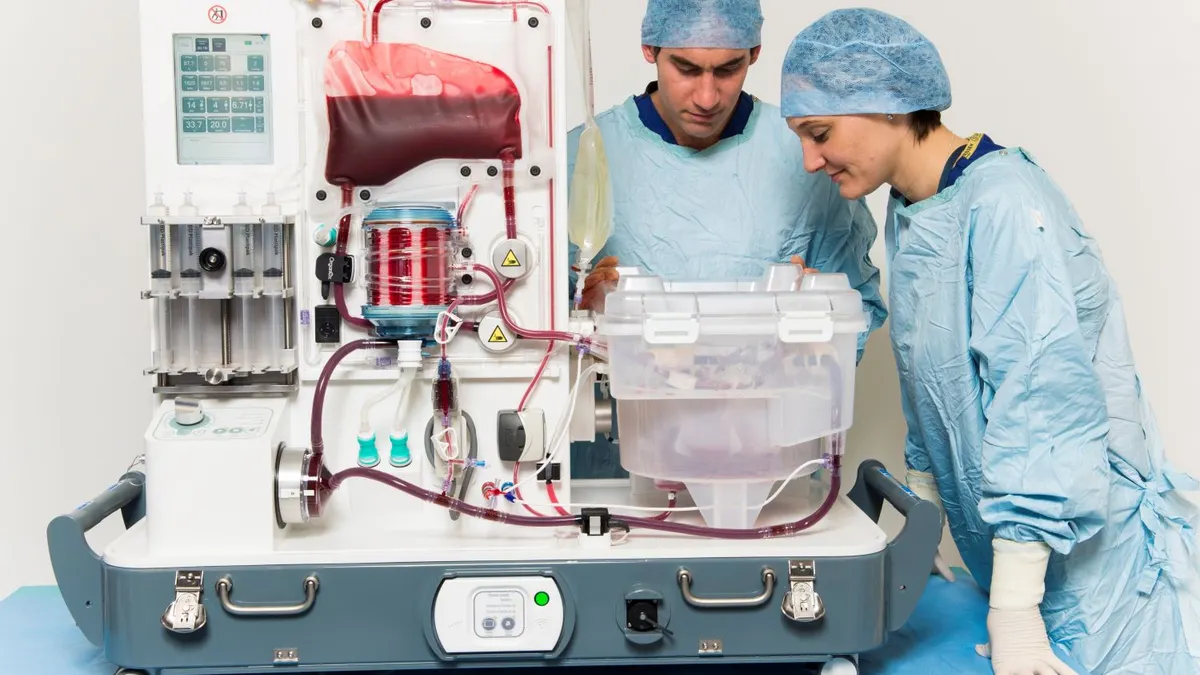
- OrganOx’s liver perfusion system supplies oxygenated blood, medications and nutrients to the donor organ near body temperature. The device allows functional assessment of the organ to support the decision on whether to transplant.
- U.K.-based OrganOx launched the system in 2022 in the U.S., where it competes with an organ preservation device from TransMedics Group. Called Metra, OrganOx’s system is also approved in the U.K., European Union, Australia and Canada.
Dive Insight:
OrganOx, founded in 2008 by University of Oxford professors Peter Friend and Constantin Coussios, generated sales of about 55.2 million pounds in 2024.
The company’s machine perfusion technology is intended to preserve organs longer and enable real-time monitoring of the organ’s condition during storage and transportation to improve transplant success rates, including by making more effective use of organs from marginal donors.
Terumo, which operates cardiac and vascular, medical care, and blood and cell technologies businesses, said the organ transplantation field, which faces donor shortages relative to demand, has significant unmet medical needs and strong growth potential. Terumo has collaborated with OrganOx over the years and in March made a strategic investment in the company through Terumo Ventures, its venture capital arm.
Needham analyst Mike Matson said it's unclear what strategy Terumo will pursue with OrganOx, but the company could continue to sell Metra to hospitals and organ procurement organizations, partner with third-party logistics providers or add its own logistics capabilities to compete with TransMedics.
“We continue to see potential for OrganOx to capture increasing share in the liver transplant market,” the analyst wrote on Monday.
The OrganOx device has been used in more than 6,000 liver transplants worldwide to date, according to the company.