UPDATE: May 16, 2024: An Abbott spokesperson wrote in an emailed statement that patients living with a Heartmate system are at “no risk” because of the issue related to the most recent recall.
“While rates of serious adverse events related to this issue are extremely low, Abbott is working with physicians to reiterate surgical instructions that can stop blood and air leakage prior to completing an LVAD implant,” the spokesperson wrote. “We are also developing a change in the seal interface for the HeartMate 3 that will help address this issue.”
Dive Brief:
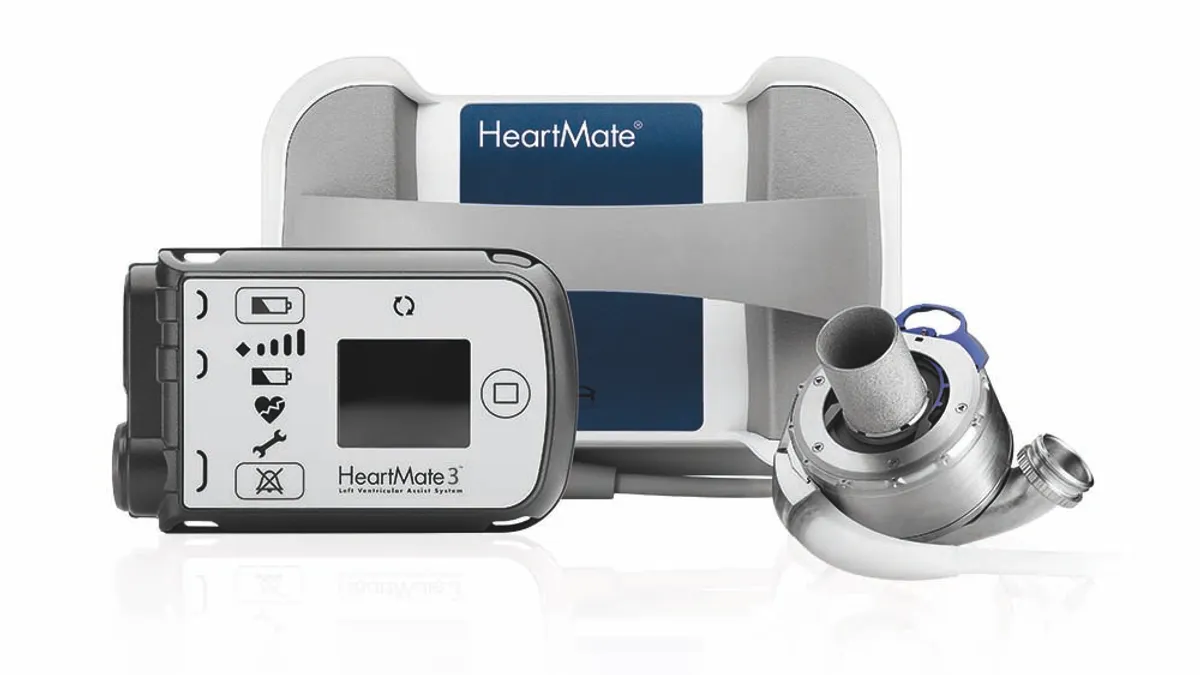
- Abbott has recalled the Heartmate 3 left ventricular assist system after a review of complaints identified blood leakage or air entering the seal between device components.
- Abbott reported 81 incidents, including 70 injuries and two deaths, related to the problem, the Food and Drug Administration said Wednesday in a recall notice. The agency labeled the recall a Class I event, the most serious type, but the action does not necessitate product removal.
- It is the third recall related to the Heartmate pump the FDA has posted since March. In April, the agency said Abbott was recalling thousands of Heartmate II and Heartmate 3 systems due to the potential for buildup of biological material that could obstruct the devices. Abbott also recently recalled a Heartmate communication system.
Dive Insight:
The Heartmate pump is used to support blood circulation in patients with severe left ventricular heart failure.
The system takes over the pumping function of the left ventricle, the heart’s main pumping chamber. Heartmate can serve as a bridge to a heart transplant or help the heart recover, and the device is used both in and outside of the hospital.
The issue of blood leakage or air entering the device was observed during implantation in all of the reported complaints, the FDA said. The problem impacts the integrity of the blood flow and may lead to longer-than-expected surgery, hemorrhage, right heart failure or air embolism.
The agency said 882 devices distributed from March 1, 2021, to the present are being recalled for the issue in the U.S. Abbott began the recall on March 13 and issued an urgent medical device correction notification letter to customers on March 20.
In its letter to customers, Abbott recommended healthcare providers follow standard surgical processes and the device’s existing instructions for use if blood leak or air entrapment is suspected or observed. The company said not to return unused devices because they are not being removed from the field.
Abbott’s previous recall of Heartmate devices, which the company began in February, was tied to reports of 273 injuries and 14 deaths and involved 13,883 devices in the U.S. In January, the company recalled a communication system that monitors patients implanted with the Heartmate 3 system because it could cause the pump to stop or start unintentionally.
Abbott became the only supplier of LVADs in the U.S. after Medtronic pulled its Heartware pump from the market in June 2021. The FDA advised healthcare providers to use Abbott’s Heartmate 3 as an alternative.