Dive Brief:
- Abbott said Monday that it has received Food and Drug Administration approval for its Volt pulsed field ablation system.
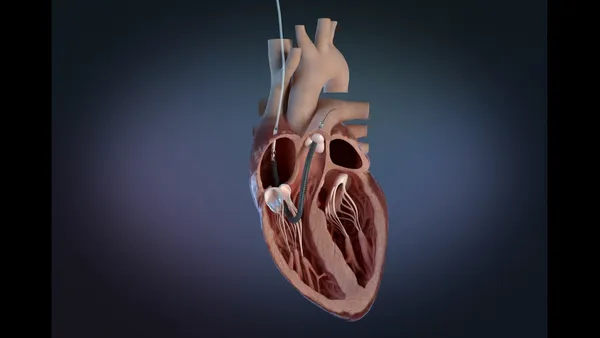
- The catheter-based device uses targeted, high-energy electrical pulses to treat a common heart arrhythmia called atrial fibrillation. Abbott’s Volt device is indicated for both paroxysmal AFib, where episodes come and go, and persistent AFib, or episodes that last longer than seven days, according to the FDA.
- Medtronic, Boston Scientific and Johnson & Johnson have all debuted their own PFA devices in the last two years. The approval allows Abbott to join the fast-growing, competitive market in the U.S.
Dive Insight:
Despite the earlier movement by competitors in PFA, Abbott CEO Robert Ford told investors in October that the company is “right on time, and we’re complete with the full portfolio.”
The company hopes that unique features in its Volt system will help it stand apart from other PFA treatments. Abbott’s device uses a balloon-in-basket design and can integrate with a 3D mapping system.
The combination is designed to help achieve durable lesions with fewer pulses, minimizing the risk of complications. It also provides the option for patients to be placed under conscious sedation instead of general anesthesia.
"We heard the physician feedback that patients need an alternative to general anesthesia during a PFA ablation procedure that doesn't sacrifice strong outcomes," Christopher Piorkowski, chief medical officer of Abbott's electrophysiology business, said in a statement.
Abbott received FDA approval based on a study of 392 people across 40 centers in the U.S., Europe, Canada and Australia. The results demonstrated meaningful performance in both paroxysmal and persistent AFib.
Abbott received Europe’s CE Mark for its Volt system earlier this year. With the FDA nod, Abbott plans to expand its device into the U.S. and across more sites in the European Union.
Meanwhile, competitors are looking to grow their existing PFA platforms. This summer, Boston Scientific received an expanded label to use its device to treat persistent AFib, and in November, Medtronic reported that its PFA sales had grown more than 300% year over year in its fiscal second quarter.