Dive Brief:
-
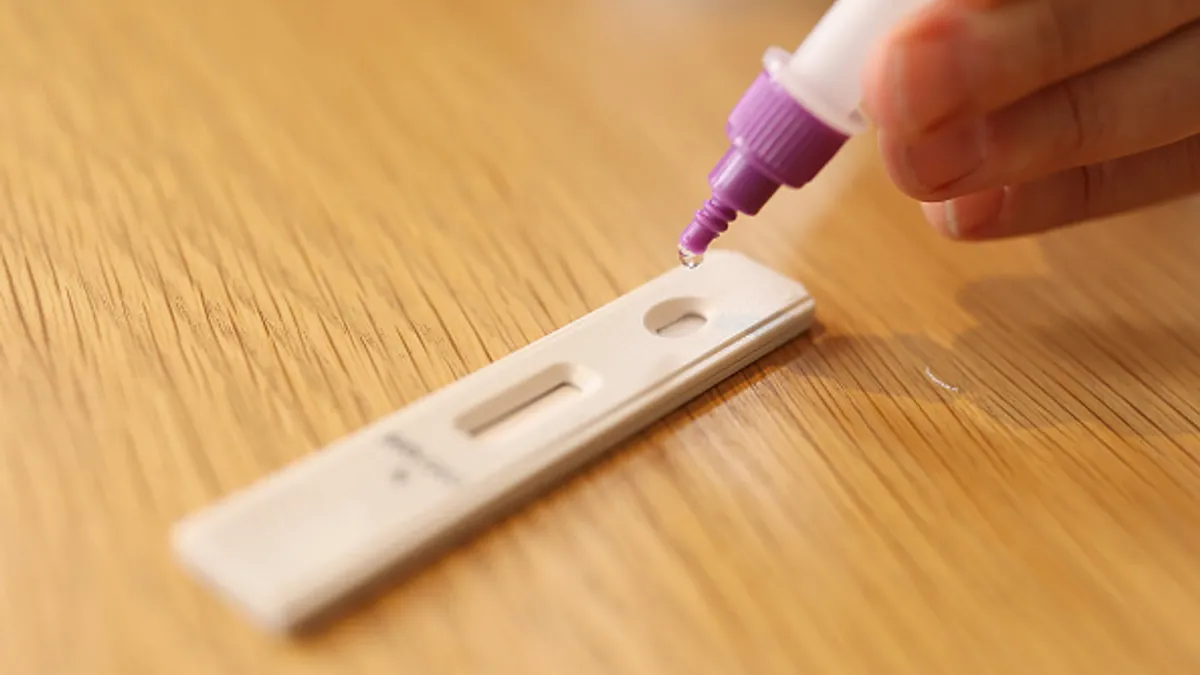
A single antigen test may only be able to correctly identify the virus 60% of the time in patients who have the omicron variant and who display symptoms of the disease, Tim Stenzel, director of the Food and Drug Administration’s Office of In Vitro Diagnostics and Radiological Health, said during a Wednesday meeting on testing.
-
The FDA is seeing an increase in samples with the omicron variant that have a relatively low viral load, also referred to as a low positive. “Instead of seeing the usual 10% to 20% low positives in clinical studies last year, we saw a jump to 30% to 40% low positives,” Stenzel said. “When you have 40% low positives… you’re going to see a really big hit in sensitivity.”
- The lower sensitivity means people testing for Covid should use multiple antigen tests to rule out a negative result, with 24 to 48 hours between tests, according to the regulator.
Dive Insight:
The FDA cautioned in late 2021 after the omicron variant emerged that antigen tests may have reduced sensitivity, meaning they correctly identify fewer people with COVID-19. Data have continued to accumulate that the tests are not as sensitive to the newer variants of the virus, Stenzel said.
“What’s actually driving that is less clear,” he added.
Most at-home antigen tests already on the market currently require multiple tests to rule out a potential COVID-19 infection. For instance, Abbott Laboratories’ BinaxNOW requires two tests, at least a day apart.
“The majority of antigen tests on the market are labeled that way, but I know some people haven’t been using them that way,” said Christina Silcox, research director for digital health at the Duke-Margolis Center for Health Policy. “Even for tests that were able to get single-use labeling, for this particular variant, I'd go ahead and start using them serially as well.”
Silcox suggested that people who have typical COVID-19 symptoms but test negative twice might consider a third test and should wear a mask and isolate if they feel unwell.
”Even if you’re testing negative, just have a little suspicion around that negative,” she said.
New antigen tests being reviewed by the FDA likely will require multiple tests, even for people with symptoms. In templates for emergency use authorizations, the agency recommends that developers validate their tests across the range of possible viral loads, including a certain percentage of low positives.
“It is challenging, and it is one of the reasons why right now it’s taking longer to review antigen test applications,” Stenzel said, referring to the lower viral load.
When the omicron variant emerged last year, test-makers said it didn’t affect their PCR tests, which screen for the presence of viral RNA. Abbott and Becton Dickinson Co. also said it didn’t affect their rapid antigen tests.
So far, the FDA has flagged two PCR tests that are expected to fail to detect the variant, but hasn’t called out any specific antigen tests.
Silcox said while PCR tests may be more sensitive than antigen tests, they take longer to deliver a result, so using multiple antigen tests may yield a similarly reliable result in the same amount of time.
In total, the FDA has authorized almost 500 different COVID-19 tests. So far, PCR tests have been cleared through the FDA’s de novo pathway, but no antigen tests have yet gone through traditional regulatory review. The agency has signaled that test-makers should start to seek clearance for their tests through traditional pathways before the public health emergency ends.
In the meantime, COVID-19 cases have been increasing across the U.S. More than 100,000 cases and 300 deaths are being reported daily, according to the Centers for Disease Control and Prevention.