Dive Brief:
- Medtronic won FDA approval for an implantable system to treat pulmonary arterial hypertension that delivers the drug Remodulin (treprostinil), developed by United Therapeutics.
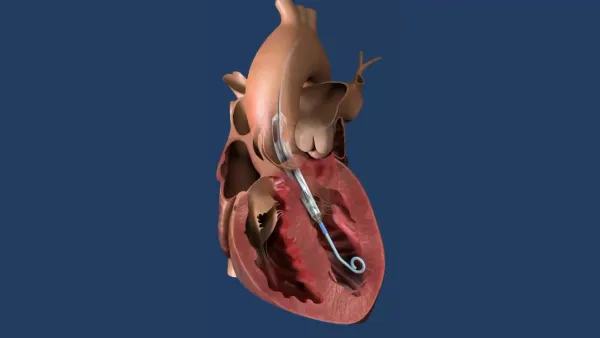
- The system consists of Medtronic’s SynchroMed II implantable drug infusion pump and a newly developed intravascular catheter. It delivers Remodulin intravenously to patients who previously received the drug via an external infusion pump.
- Called the Implantable System for Remodulin, the system is indicated for adult patients with Class I, II and III pulmonary arterial hypertension receiving intravenous delivery of the medication.
Dive Insight:
Pulmonary arterial hypertension is a rare and serious form of a broader condition known as pulmonary hypertension, or high blood pressure in the lungs. It occurs when the very small arteries throughout the lungs narrow in diameter, increasing the resistance to blood flow. Increased blood pressure over time can damage the heart.
The condition affects about 15 to 50 people per million in the United States, according to the American Lung Association. Because patients with pulmonary arterial hypertension often are not diagnosed until their symptoms are severe, they may only have a few years to live unless they get proper treatment, the group said.
"External infusion pumps have been used to deliver prostacyclins for PAH, but managing the therapy places a significant burden on patients, interferes with their daily activities, and runs a high risk of infections," David Steinhaus, general manager of the heart failure business at Medtronic, said in a press release.
FDA approval was based on the DelIVery for PAH trial that enrolled 64 patients (60 successfully implanted) and showed the implantable intravascular delivery system effectively delivered treprostinil, with a low rate of catheter-related complications, Medtronic said.
During the pivotal clinical trial, 10% of patients experienced pump failures after four years of use, the company said. At least 33% of these failures resulted in the device failing to deliver Remodulin without sounding an error alarm. The remaining malfunctions occurred with a motor stall alarm that was reported by the patient. Patients who cannot tolerate a sudden cessation of Remodulin therapy, or who have hearing loss, may not be appropriate candidates for the implantable system, Medtronic said.
Medtronic and United Therapeutics pursued parallel regulatory filings for the device and drug. United Therapeutics will lead the commercial promotion of the system.