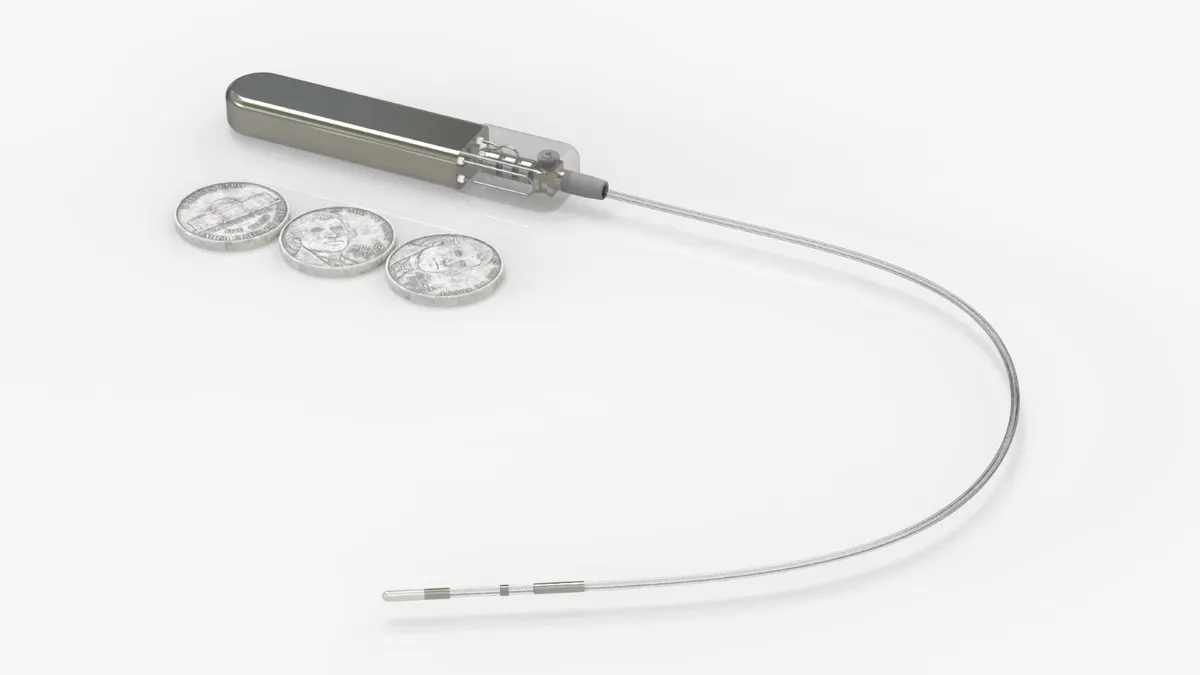
Glucotrack, a startup making an implantable glucose monitor, shared data from a first-in-human trial in June during the American Diabetes Association’s Scientific Sessions. The 10-person study, where patients were observed inpatient for four days and for a week after device removal, met safety and performance endpoints.
CEO Paul Goode said in an interview that the goal of the study was to show that the device and the procedure to implant it were safe.
No serious adverse events were reported during the study. For accuracy, the results showed a mean absolute relative difference of 7.7%, which would make the device competitive with CGMs on the market, although Goode cautioned that the data is very early and the results were not statistically significant. MARD is a measurement that compares blood glucose readings from a CGM to reference measurements, such as blood glucose from a fingerstick.
Glucotrack is taking an implantable device approach to address some of the challenges that people experience with wearable CGMs, Goode said, such as the lag time in readings when blood glucose changes quickly and irritation from adhesives.
“If you look at CGMs, they're wonderful products. Many of us in the company have worked on one or more of those generations of products, and they've done a lot of good for patients with diabetes,” said Goode, who has worked for Dexcom and MiniMed, the diabetes tech business that was later acquired by Medtronic. “That being said, there are still common problems.”
Most continuous glucose monitors on the market today are worn on the skin and measure the concentration of glucose in the interstitial fluid surrounding cells, which is then used to calculate blood glucose values. Glucotrack’s device is different in that it is implanted in the subclavian vein near the collarbone and measures glucose values directly from the blood. Goode said the approach to implantation is similar to a minimally invasive procedure for placing a pacemaker — the CGM is placed two inches into the vein instead of into the heart.
Glucotrack intends for its sensor to last for three years, although it will still need to prove that out in a study.
Senseonics, which currently has the only implantable CGM on the U.S. market, received Food and Drug Administration clearance last year for the device to be worn for a year. Goode said the development is good for patients, adding that he expects Glucotrack’s implant to last longer because of differences in the devices’ placement and chemistry.
Senseonics’ device sits in the upper arm, in the subcutaneous space, “which is the most difficult environment to get something to work,” Goode said, while Glucotrack’s device sits in the bloodstream.
Glucotrack is approved to do another study in Australia with 30 people that will test the implanted CGM for a year. The company is also working with the FDA on a presubmission, Goode said, with hopes of starting a study in the U.S. next year.