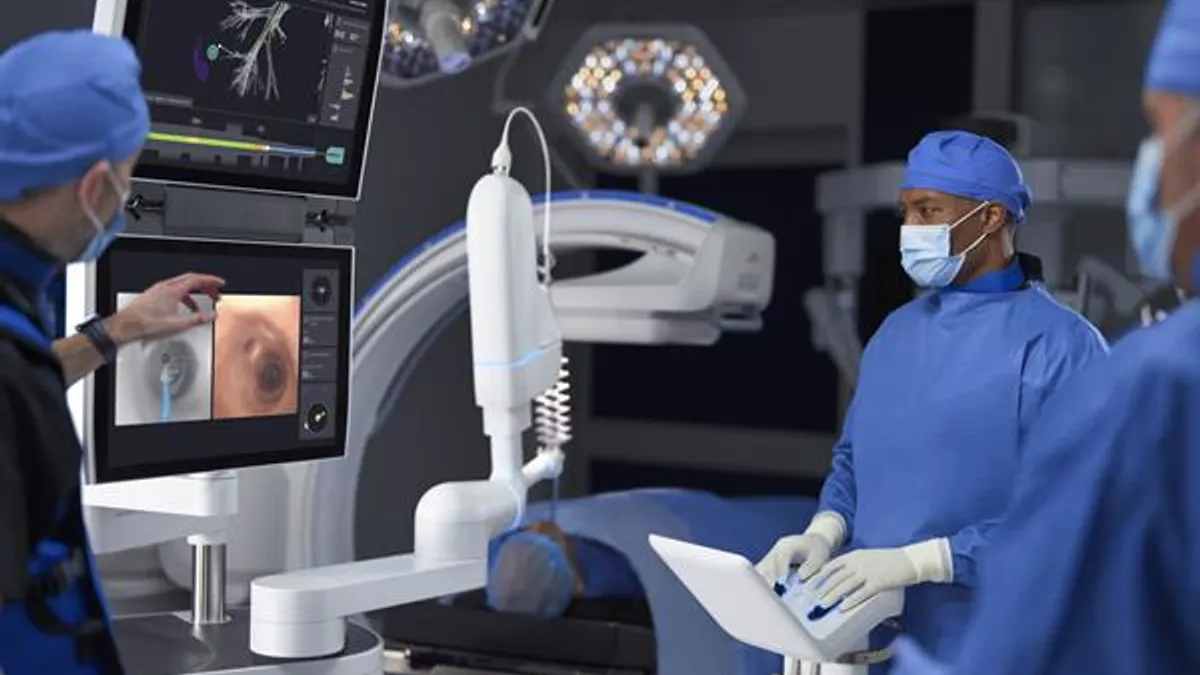
Intuitive Surgical said Wednesday its Ion system received Food and Drug Administration clearance for software that integrates artificial intelligence for navigation in lung biopsy procedures and improves imaging capabilities.
The robot-assisted bronchoscopy system is designed to help physicians reach small nodules deep in the lungs and position biopsy tools to sample the tissue.
The AI enhancement addresses a challenge that occurs when a lung nodule shifts to a different location than in the pre-procedure CT scan. Comparing live images to the original plan, the system adjusts the view along the navigation path.
Intuitive will introduce the software in a limited launch to gather insights into performance across a range of environments, ahead of a broader U.S. rollout planned for 2026.
As of June 30, more than 900 Ion systems have been placed in hospitals across 10 countries, Intuitive said in the announcement.
Horizon performs first cataract surgery
Horizon Surgical Systems said the first cataract surgery was performed with its robot-enabled system designed for ophthalmology procedures.
Los Angeles-based surgeon Uday Devgan performed the procedure.
Horizon said the system, called Polaris, aims to reduce variability and increase precision in cataract surgery.
“With Polaris, I experienced a new level of control that directly addresses those challenges,” Devgan said in Horizon’s statement on Wednesday. “It has the potential to deliver more reliable outcomes for patients.”
The company said it expects to treat additional patients in the coming months as it works toward FDA approval and commercialization.
MMI completes robotic brain surgeries
Robotics company MMI said Monday that it completed the first cases in a neurosurgical clinical trial for its Symani system.
Adnan Siddiqui, vice chairman in the Department of Neurosurgery at the State University of New York at Buffalo’s Jacobs School of Medicine and Biomedical Sciences, performed the surgeries to restore blood supply to the brain in three adults with Moyamoya disease, a condition where the carotid artery in the skull becomes blocked or narrowed.
The investigational cases are part of an early feasibility study approved by the FDA to assess the safety and preliminary effectiveness of the Symani system for patients with Moyamoya disease.
MMI gained FDA de novo authorization last year for the system for use in soft tissue manipulation to perform microsurgery, a specialized technique that involves reconnecting tiny vessels to restore blood flow or redirect fluid during reconstruction or repair.