Dive Brief:
- Johnson & Johnson’s Abiomed has sent an urgent medical device correction about a malfunction risk linked to 22 reports of serious injuries.
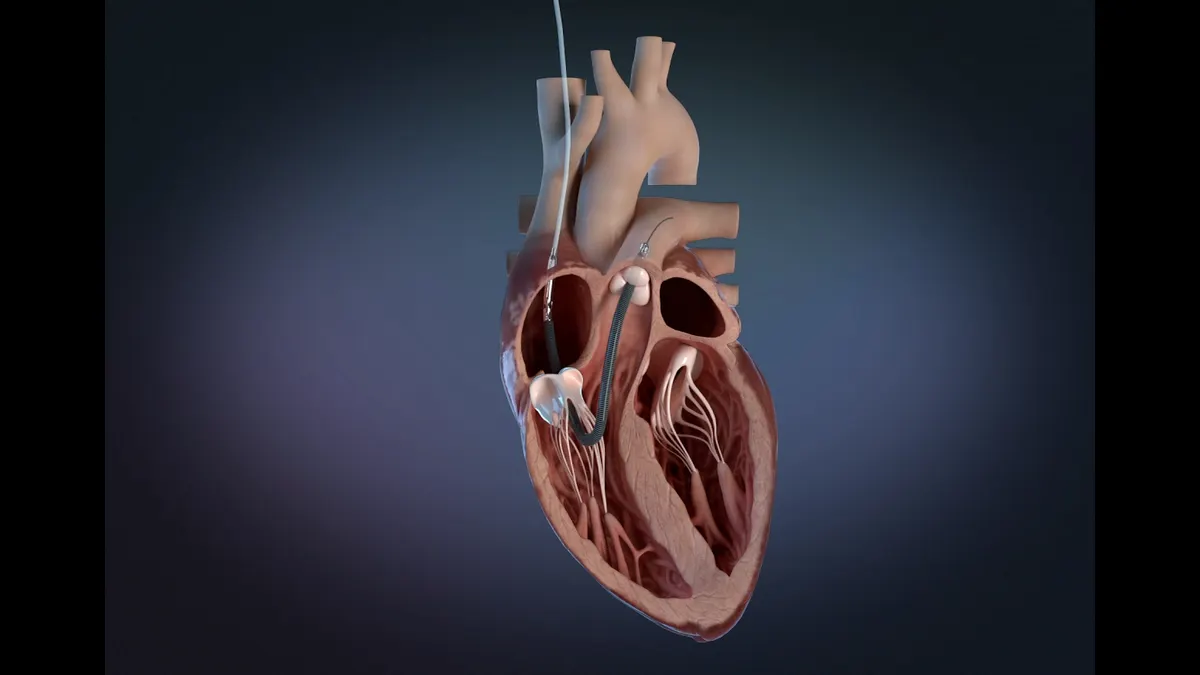
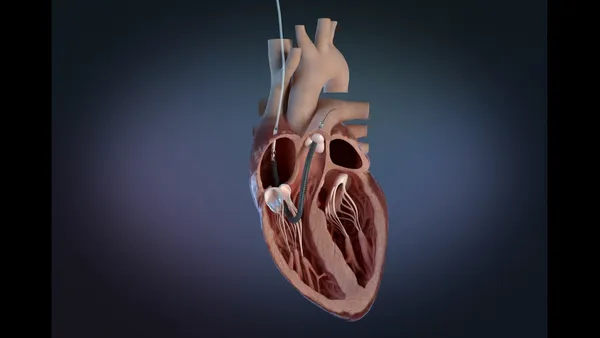
- The Food and Drug Administration, which published an early alert about the devices on Tuesday, said sensor values in Impella RP heart pumps may drift over time.
- Erroneous information on automated Impella controllers has caused users to make inaccurate adjustments to the devices and unnecessary pump exchanges, the FDA said.
Dive Insight:
J&J wrote to customers last week after learning that the differential pressure sensor in its Impella RP devices may malfunction, causing sensor values to drift over time. Sensor drift can cause inaccurate information about pump flow and other metrics. The FDA said it is impossible to confirm an error in the flow calculation directly from the pump signals when sensor drift occurs.
A J&J spokesperson shared a similar assessment of the device correction in an emailed statement. The sensor drift risk does not affect the pump’s ability to provide hemodynamic support, the spokesperson said, and hospitals can continue using Impella RP devices.
J&J is updating its instructions for use. Clinicians should continue to monitor patient hemodynamics with approved diagnostic devices and verify Impella positioning with imaging before clinical interventions, according to the FDA alert. Rather than rely on the displayed values, clinicians should refer to flow rates listed in the instructions for use.
The FDA is reviewing details of the potentially high-risk device issue and will provide updates if significant new information becomes available. Early alerts are often precursors to FDA recall notices.
J&J has written to customers about multiple issues linked to Abiomed devices in recent months. In December 2024, the company updated its instructions for use for the two Impella RP devices affected by the latest correction. The FDA published a Class I recall notice about the updated instructions in January 2025, reflecting the risk that the pumps may temporarily or permanently stop.
The recall notice was the first of a series of announcements about Abiomed devices in 2025. The FDA published recall and early alert notices about automated Impella controllers in July, August, September and October.