Dive Brief:
- Medtronic’s planned diabetes device spinoff MiniMed has filed for an initial public offering, the company said Friday.
- The filing shows MiniMed made a $198 million loss on sales of $2.72 billion in its 2025 fiscal year. MiniMed plans to grow profits by using its broad portfolio, which features both insulin pumps and glucose sensors, to generate more revenue per customer than its competitors.
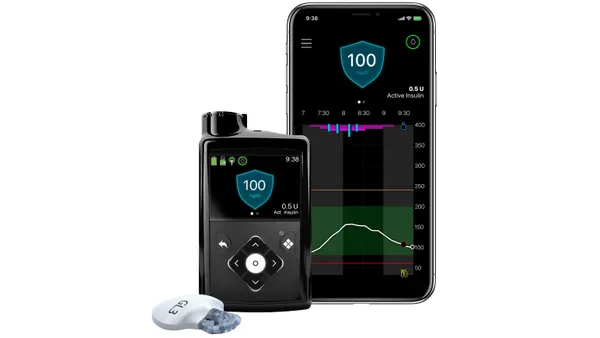
- MiniMed’s Simplera CGM sensor is key to the strategy, but the company warned it may be unable to make the continuous glucose monitor profitably amid manufacturing challenges.
Dive Insight:
Medtronic announced in May that it would spin off its diabetes business into a separate company, called MiniMed, creating a standalone competitor for diabetes tech firms like Dexcom, Insulet and Tandem Diabetes Care.
MiniMed is pitching itself to investors as the only company that sells all parts of an integrated diabetes management system. The company has a portfolio of insulin pumps and pens, CGMs and other devices that it says will support higher sales volumes and margins than rivals such as Dexcom and Insulet that only compete for part of the market.
To support the strategy, the company is developing products such as its patch pump MiniMed Fit, which will compete with Insulet’s Omnipod 5, and launching its Simplera CGM sensor. MiniMed aims to file for FDA approval of MiniMed Fit by the fall of calendar year 2026.
The Simplera CGM won FDA approval in April and launched in September. MiniMed described problems with the launch in the IPO filing. Production of Simplera CGM products is scaling more slowly than anticipated because initial high-volume automated manufacturing has underperformed. MiniMed said efforts to fix the issues and develop additional automated manufacturing lines have encountered difficulties.
The company expects to record a pre-tax charge of $100 million to $120 million in the quarter ending Jan. 23, 2026. The charge reflects unsuccessful efforts to develop high-volume automated manufacturing lines with third-party manufacturers for Simplera CGMs. MiniMed said it is unsure if it can make the CGM profitably and could face cost overruns or additional delays even if it does.
A delayed U.S. Simplera CGM launch and increased competition drove a 15% decline in U.S. insulin pump sales over the first six months of MiniMed’s 2026 fiscal year. The company said the delayed launch led to a competitive disadvantage and limited its ability to sell more new pumps in the first half of its fiscal year.
The company plans to add to the growth of its existing portfolio by striking strategic partnerships and tuck-in acquisitions. Medtronic has already partnered with Abbott on a insulin delivery system, which pairs a Medtronic insulin pump with a CGM that was developed by Abbott specifically for the partnership.
Separating from Medtronic will give MiniMed more financial flexibility, the company said, and it has identified “an attractive set of strategic opportunities across potential partnerships and tuck-in acquisitions.”