Dive Brief:
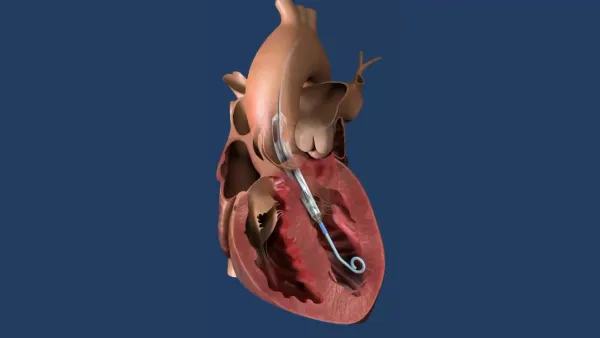
- Medtronic said it has begun a clinical study investigating a shortened dual antiplatelet therapy (DAPT) regimen in patients at high risk for bleeding who receive its next-generation Resolute Onyx drug-eluting stent (DES) implant.
- DAPT is a combination of aspirin and an anti-clotting medication. The study will assess safety risks including cardiac death, heart attack and stent thrombosis in patients in the United States and Japan who are prescribed DAPT for only one month.
- Medtronic said the clinical trial, called the Onyx One Clear Study, will look at whether one month of DAPT is enough to minimize stent thrombosis and other complications, while lowering bleeding-related risks from prolonged antiplatelet therapy.
Dive Insight:
Current guidelines recommend dual antiplatelet therapy for a minimum of 12 months after the implant of a drug-eluting stent in a percutaneous coronary intervention, according to the American College of Cardiology. But the optimal duration is unclear.
Various studies have shown the potential to safely reduce DAPT duration to three to six months. But other studies have shown benefits of of a DAPT regimen longer than 12 months, even with greater bleeding risk. One study found reduced risks of major complications in DES patients prescribed one month of antiplatelet therapy, compared with those who got bare-metal stents.
"A major clinical question that remains is whether we can further reduce the mandatory duration of dual antiplatelet therapy after DES implantation," Ajay Kirtane, director of New York-Presbyterian Hospital/Columbia University Cardiac Catheterization Laboratories, said in a press release.
Gregg Stone, professor of medicine at Columbia University, said one-month DAPT duration in high-bleeding-risk patients has the potential to substantially improve the safety of stent procedures for this group.
Medtronic said the Onyx One Clear Study and a companion trial that began enrollment in late 2017 called the Onyx One Global Study will evaluate about 2,700 patients in total in up to 140 sites worldwide.
“This large-scale clinical study will help address an unmet need as high bleeding risk patients have been largely underrepresented in previous studies looking at shorter DAPT duration," said David Kandzari, director of interventional cardiology and chief scientific officer at Piedmont Heart Institute in Atlanta in a statement.
Medtronic’s Resolute Onyx DES received Europe's CE mark in September 2014 and FDA approval in April 2017.
Abbott Laboratories is currently studying whether three months of DAPT is non-inferior to the standard 12-month duration in 2,000 high-bleeding-risk patients who have received its Xience drug-eluting stent.