Dive Brief:
- Senseonics filed a submission with the Food and Drug Administration that will allow it to integrate its implantable glucose monitor with insulin pens and pumps, CEO Tim Goodnow said in a Thursday call with investors.
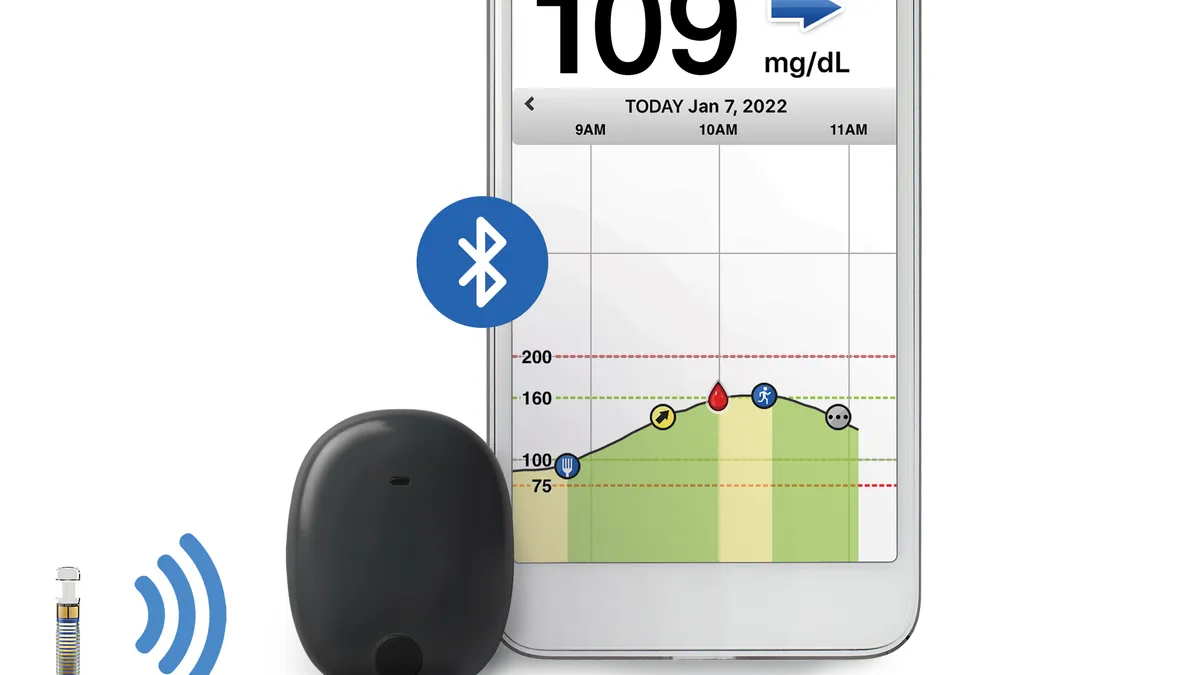
- The Germantown, Maryland-based company makes a continuous glucose monitor, called Eversense, that is implanted under the skin for up to 180 days, with an external transmitter that sends results to users’ smartphones.
- The company is also running a trial of a longer-duration version of its sensor that could be worn for a full year.
Dive Insight:
Senseonics expects approval for the iCGM designation in early 2024, Goodnow told investors. The CEO did not disclose which pump companies Senseonics will partner with, noting that the company “has a number of development programs currently in place,” and that insulin pens are also an important consideration, given that more people use them.
Senseonics is also nearing the end of its Enhance trial, which is designed to evaluate the accuracy and safety of a version of its sensor that can be worn for 365 days. Goodnow said the last patient evaluation visits will be finished in September. The company plans to make an FDA submission for the longer-duration sensor in early 2024, BTIG analyst Marie Thibault wrote in a research note on Thursday.
“While we like the Eversense technology, we cannot yet say with certainty that adoption trends are nearing an inflection point,” Thibault wrote. “We think a longer-duration sensor and iCGM designation will make the technology more appealing to a broader segment of the population, but until we see robust, sustained adoption of Eversense, we remain skeptical of the steep step-up in sales projected for 2024 and beyond.”
Senseonics reported $4.1 million in revenue in the second quarter, an 11% increase year-over-year. The company reported a net loss of $20.4 million in the quarter, compared to net income of $104.2 million in the year-ago period.
It expects sales of $20 million to $24 million for the full year.