Dive Brief:
-
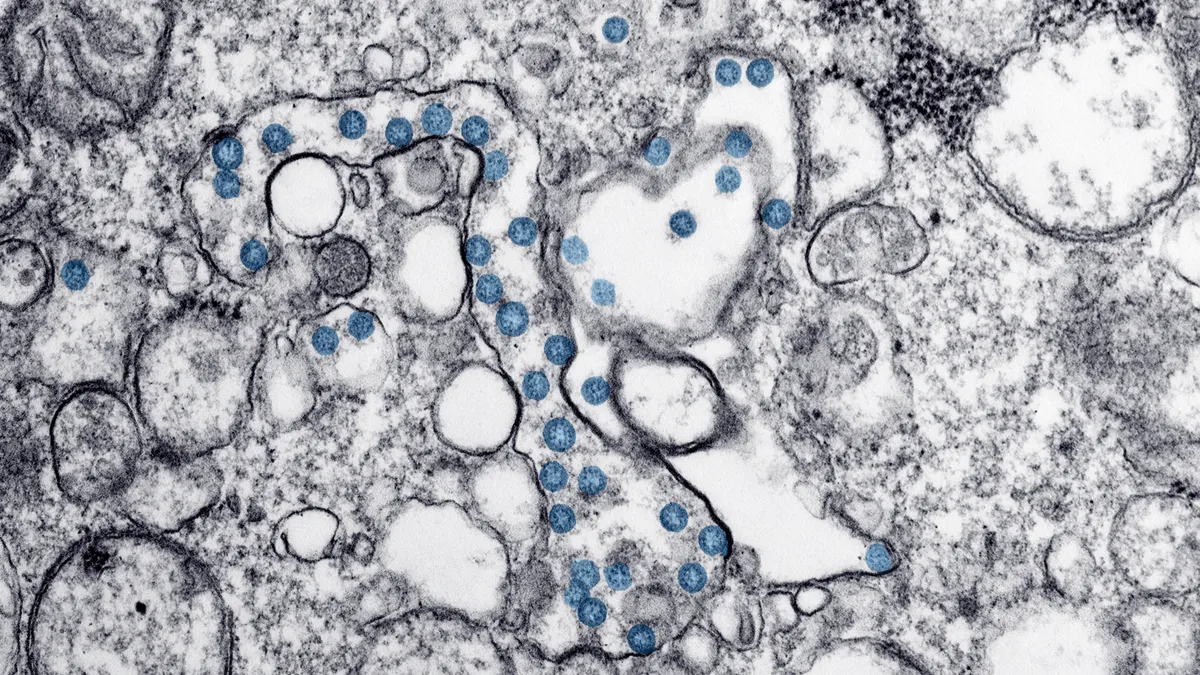
FDA flagged the risk of both false positive and negative results with the Thermo Fisher Scientific TaqPath COVID-19 Combo Kit in a letter to lab staff and healthcare providers on Monday.
-
The agency said issues relating to the test kit and associated software can cause the molecular SARS-CoV-2 assay to generate inaccurate results.
-
Thermo Fisher said it's working with FDA to resolve the issues, and updated the software and instructions. FDA is still advising laboratories to assess all positive results to see if the samples need retesting.
Dive Insight:
The warning appears to be the second of its kind to health providers tagging potential false results with a coronavirus test, coming after last month's letter noting BD's coronavirus test saw 3% of results in one study as false positives.
In May, FDA noted concerns about the accuracy of Abbott’s ID Now test. In that case, FDA responded to a study that linked that test to a higher rate of false negatives than rival COVID-19 tests.
In this latest case, FDA granted emergency use authorization to TaqPath in March, one of the first. Since then, Thermo Fisher has acquired modifications and the test has been used in studies to support other EUAs, including the Yale University saliva test that FDA OK'd for use over the weekend.
Thermo Fisher received the EUA based on a study showing TaqPath correctly detected the coronavirus in 100% of nasopharyngeal swab and bronchoalveolar lavage samples. However, FDA has now alerted laboratories and healthcare providers to the risk of inaccurate results after hearing that some TaqPath users have complained to Thermo Fisher.
FDA highlighted two potential causes of inaccurate results. Firstly, Thermo Fisher found “inadequate” vortexing or centrifugation of RT-PCR reaction plates can cause TaqPath to deliver false positives. Secondly, an issue with the software can lead to false negatives or inconclusive results.
The agency is advising TaqPath customers to stop using older versions of Applied Biosystems COVID-19 Interpretive Software and read updated instructions on vortexing and centrifugation to ensure sufficient mixing. Thermo Fisher is providing support to try to ensure laboratories perform the test correctly.
“We are working with the FDA to make sure that laboratory personnel understand the need for strict adherence to the instructions for use. We strongly encourage laboratories to participate in the training that we offer to make sure all workflow processes are followed. We also provide 24-hour support for labs that are processing COVID-19 test kits,” a Thermo Fisher spokesperson wrote in an emailed statement.
Despite the changes implemented by Thermo Fisher, FDA still wants laboratories and healthcare providers to take precautions when dealing with TaqPath results. FDA recommends laboratories look at the amplification curves for all positive results to assess whether the plate was sufficiently mixed, and retest samples that may not have been.
FDA is also advising healthcare professionals to interpret TaqPath results in light of other evidence, such as clinical observations and epidemiological information.
The FDA alert follows a quarter in which COVID-19 added $1.3 billion to Thermo Fisher’s sales. Thermo Fisher has since landed a Department of Health and Human Services contract to provide 96 of its instruments to laboratories and has also had its planned takeover of Qiagen blocked by investors.