COVID-19: Page 25
-


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

MDUFA V talks kick off as FDA grapples with onslaught of COVID-19 submissions
Medtech industry groups broadly expressed a desire to maintain the status quo after FDA Commissioner Stephen Hahn described the strain on agency workers under MDUFA IV as unsustainable.
By Maria Rachal • Oct. 28, 2020 -
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
LabCorp sees Q3 revenue rise 33%, but doesn't follow Quest in upping guidance
CEO Adam Schechter said the testing giant is following its rival in returning CARES Act funds, given the spike in COVID-19 testing revenues and return of routine screenings.
By Greg Slabodkin • Oct. 27, 2020 -
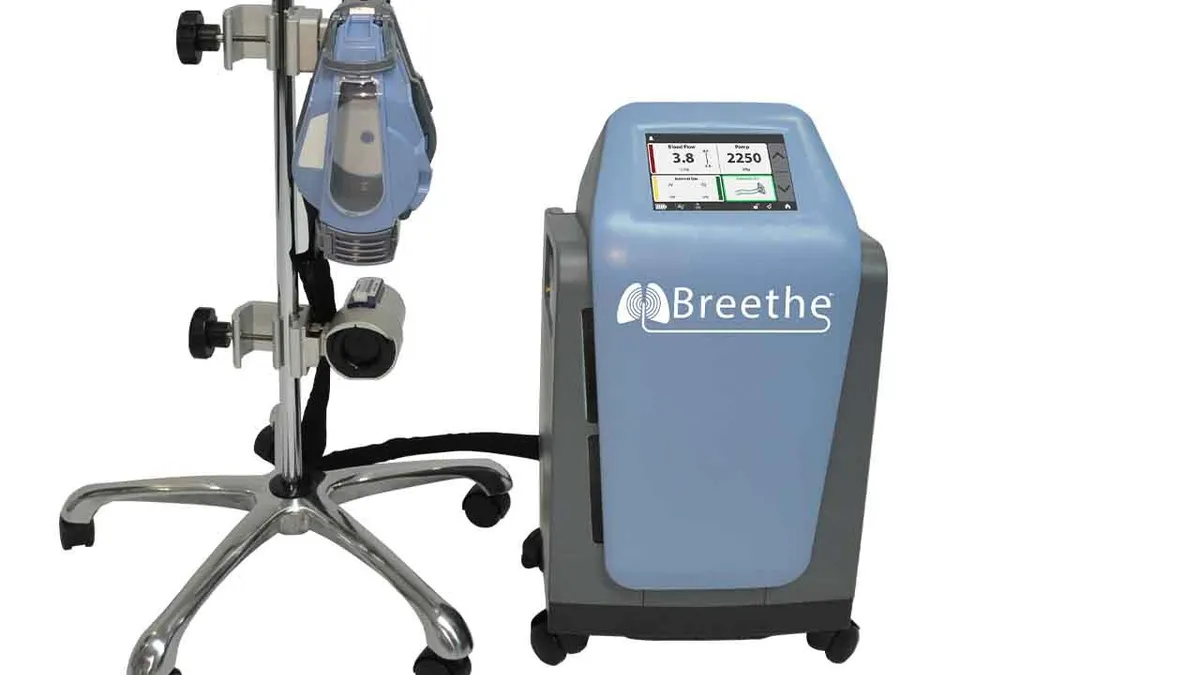
 Retrieved from Abiomed/BusinessWire on April 30, 2020
Retrieved from Abiomed/BusinessWire on April 30, 2020
FDA grants 510(k) to Abiomed's artificial lung, teeing up use in COVID-19
While LivaNova and Medtronic already sell ECMO devices, Abiomed is focusing on a relatively compact design to differentiate the device from the pack.
By Nick Paul Taylor • Oct. 27, 2020 -
 Retrieved from Pixabay.
Retrieved from Pixabay.
Despite recent rebound, hospitals find admissions still well below pre-pandemic levels
Trends from health systems in New York and California suggest patients may have lost health insurance as a result of the economic fallout from the pandemic
By Ron Shinkman • Oct. 26, 2020 -
Medtech Q3 results signal diagnostics boom, mixed bag on device growth
Hologic, BD and Zimmer Biomet are among the medtechs set to add to the growing mass of late summer data points this week.
Updated Nov. 2, 2020 -
HHS walks back CARES fund reporting requirement hospitals feared
Health systems worried they may lose COVID-19 relief money after guidance changed last month. Those funds have been crucial in mitigating big hits to capital spending, execs at medtechs like BD and Stryker have said.
By Samantha Liss • Oct. 23, 2020 -
Quest revenue jumps 43% on COVID-19 tests, hands back federal funds
Execs said they now have a "clearer line of sight" into base business and coronavirus testing trends, but both remain "swing factors." Still, the lab giant raised its 2020 outlook and is returning $138 million in CARES monies.
By Greg Slabodkin • Oct. 22, 2020 -
Abbott device growth returns as Wall Street queries staying power of COVID-19 test boom
CEO Robert Ford argued that focusing on how coronavirus test demand will evolve "misses the point," touting the longer-term benefits of its now-expanded diagnostic platforms footprint.
By Maria Rachal • Oct. 21, 2020 -
Thermo Fisher's coronavirus tailwinds swell, outpacing Q3 analyst estimates
Sales of PCR test kits, instrumentation and viral transport media are all growing quickly enough to offset declines in the price of personal protective equipment and ongoing pressures on immunodiagnostics and transplant diagnostics.
By Nick Paul Taylor • Oct. 21, 2020 -
FDA, medtechs envision new era for clinical trials amid COVID-19 shake-up
Medtronic, for example, has faced "very significant delays" in getting products approved as a result of research disruptions, the company's VP of global clinical affairs said during a discussion at TCT.
By Maria Rachal • Updated Oct. 20, 2020 -
Future of COVID-19 products, device shortages, CDS top CDRH 2021 to-do list
Regulators aim to publish final guidance on five topics and draft documents on another dozen priority areas in the coming year. A few of the goals are carryovers as priorities shifted during the public health emergency.
By Susan Kelly • Oct. 19, 2020 -
Philips Q3 connected care sales up 42%, but ventilators to decline next year
The company expects the COVID-19 tailwinds, including double-digit growth in patient monitors and ventilators, to extend through the fourth quarter but fall away in 2021.
By Greg Slabodkin • Oct. 19, 2020 -
CMS to cut COVID-19 test pay by 25% for delayed results, labs cry foul
The American Clinical Laboratory Association, which includes LabCorp and Quest, argued the new policy fails to address the root of delays: fluctuating demand and supply chain disruptions.
By Nick Paul Taylor • Oct. 16, 2020 -
Intuitive sees partial Q3 recovery but warns COVID-strained hospital finances continue
Between customers tightening their belts and having first-time competition on the horizon, the longtime robotic surgery leader is rethinking approaches to pricing and M&A.
By Nick Paul Taylor • Oct. 16, 2020 -
Roche diagnostics Q3 sales spiked 18% as COVID-19 offset routine test drop
Execs contend the momentum is sustainable even with arrival of a vaccine, citing government demand for high-throughput machinery and a shift in importance to antibody testing.
By Maria Rachal • Oct. 15, 2020 -
Qiagen beats sales forecast on COVID-19 growth, but leaves room for skeptics
Non-coronavirus revenue saw a mid-single-digit drop and sales of a key TB test fell 20%. While those metrics improved from earlier in the year, Cowen analysts said the slump gives ammunition to doubters.
By Nick Paul Taylor • Oct. 14, 2020 -
J&J US device unit returns to growth, potential bellwether for medtech Q3 earnings
A double-digit rise in interventional sales was offset by ongoing headwinds in parts of the orthopaedic and surgery franchises.
By Nick Paul Taylor • Oct. 13, 2020 -
Cancer testing drives 2020 medtech IPO surge after slow start
A flurry of activity began in June, including from China-based Burning Rock and Genetron and U.S.-based Progenity.
By Nick Paul Taylor • Oct. 12, 2020 -
5 key exec insights from 2020's MedTech Conference
Top leaders from Abbott, BD, Medtronic, Stryker and Zimmer Biomet shared the latest on the industry's recovery during the pandemic and thoughts on where it goes from here.
By Maria Rachal • Oct. 9, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Roche, Abbott, LDTs are most used COVID-19 tests as supply chain constraints force labs to diversify
Shortages of test kits and other supplies persist, according to the labs polled by the Association for Molecular Pathology.
By Nick Paul Taylor • Oct. 9, 2020 -
Fresenius sees COVID-19 driving dialysis device growth, eyes home care strategy
Execs at the company, which upped production of machines by 230% in response to the crisis, laid out plans at an investor day Thursday.
By Nick Paul Taylor • Oct. 9, 2020 -
FDA takes hands off EUA review for COVID-19 lab developed tests
The move follows the administration in August no longer requiring premarket review for LDTs, letting labs voluntarily seek emergency use nods. A lab trade group said the latest decision creates "unnecessary confusion."
By Greg Slabodkin • Oct. 8, 2020 -
Abbott, on defense, details embattled rapid COVID-19 test results
New data on the ID Now product comes amid criticism that the White House leaned too heavily on the quick-turnaround diagnostic, in light of President Donald Trump's illness and widespread West Wing infection.
By Nick Paul Taylor • Oct. 7, 2020 -
'Tragedy' if FDA doesn't extend COVID-19 lessons beyond pandemic, Shuren says
Device head Jeff Shuren acknowledged the regulatory paradigm for medical devices is more than 40 years old and "not well suited for many modern-day technologies," speaking at AdvaMed's Virtual MedTech Conference.
By Greg Slabodkin • Oct. 7, 2020 -
Amid pandemic, specialty telehealth outpaces urgent care, Amwell poll says
Willingness to use virtual care notably doubled in a handful of high-volume fields like radiology, cardiology and surgery, the survey found.
By Rebecca Pifer • Oct. 6, 2020









