Diagnostics: Page 3
-
Quest to offer Fujirebio’s FDA-cleared Alzheimer’s blood test
The company plans to make the diagnostic available to physicians and researchers this summer, expanding its portfolio beyond laboratory developed tests.
By Nick Paul Taylor • July 15, 2025 -
Waters, BD biosciences unit agree to $17.5B merger
The BD business will be spun off and merged with a Waters subsidiary.
By Susan Kelly • July 14, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Sara Silbiger via Getty Images
Sara Silbiger via Getty Images Trendline
TrendlineTop 5 stories from MedTech Dive
From haphazard layoffs at the Food and Drug Administration to the industry’s current IPO environment and tracking FDA-authorized AI devices, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Danaher appoints Martin Stumpe as chief technology and AI officer
The company said Stumpe’s promotion is a “pivotal step in [its] digital transformation and its ambition to lead the next era of innovation in life sciences and diagnostics.”
By Nick Paul Taylor • June 30, 2025 -
MedTech Europe urges EU to exclude devices, diagnostics from trade war
MedTech Europe said the EU’s list of products it could target in retaliation for U.S. actions “includes over 800 trade codes related to medical technologies.”
By Nick Paul Taylor • June 23, 2025 -
Illumina to buy SomaLogic for up to $425M
Illumina said adding SomaLogic would support the company’s multiomics strategy and strengthen the value of its NovaSeq X products.
By Susan Kelly • June 23, 2025 -
Could the FDA take an indirect approach to regulate LDTs?
Attorneys said many questions remain about LDT regulation after the FDA lost its legal fight. A recent warning letter could be a clue to future enforcement.
By Susan Kelly • June 16, 2025 -
Quest partners with MD Anderson to develop cancer risk blood test
Quest plans to refine, develop and validate the technology and is aiming to launch the test in North America next year.
By Nick Paul Taylor • June 13, 2025 -
Caris Life Sciences plans $424M IPO
Caris, which offers tools for disease diagnosis, therapy selection and treatment monitoring, is the latest company to test the medtech IPO market.
By Susan Kelly • June 10, 2025 -
Freenome posts pivotal liquid biopsy data as FDA filing nears completion
Freenome plans to complete all modules of its premarket approval submission in mid-2025.
By Nick Paul Taylor • June 4, 2025 -
Clairity receives FDA OK for breast cancer risk prediction tool
The startup received de novo authorization for the AI tool, the first of its kind that analyzes mammogram images to predict breast cancer risk over five years.
By Elise Reuter • June 3, 2025 -
FDA declines to appeal court order that stopped LDT final rule
“The Trump Administration has effectively ended the Biden Administration's attempt to regulate lab-developed tests,” TD Cowen analysts wrote.
By Susan Kelly • June 3, 2025 -
AstraZeneca drug could help keep a common breast cancer at bay
Data presented at ASCO show that swapping in the oral drug camizestrant for an older therapy helped sustain the benefit of initial treatment, potentially opening a novel step in patient care.
By Ned Pagliarulo • June 2, 2025 -
Teal posts data on newly approved at-home cancer screening device
Self-collection correctly identified 95.2% of HPV samples that are associated with increased cancer risk.
By Nick Paul Taylor • May 20, 2025 -
FDA clears first blood test to aid Alzheimer’s diagnosis
The FDA cleared the test for the early detection of amyloid plaques associated with Alzheimer’s in people aged 55 years and older with signs and symptoms of the disease.
By Nick Paul Taylor • May 19, 2025 -
SEC ends probe of Illumina’s Grail acquisition
The DNA sequencing company also cut its 2025 earnings outlook to reflect the impact of tariffs on its business.
By Susan Kelly • May 9, 2025 -
Earnings & Tariffs
Danaher predicts $350M tariff hit, moves to mitigate impact on profits
The company is planning manufacturing footprint changes, supply chain adjustments, surcharges and cost cutting to limit the impact of the tariffs.
By Nick Paul Taylor • April 24, 2025 -
Illumina, Tempus AI partner to drive genomic testing beyond cancer
The partners plan to build evidence packages that standardize comprehensive genomic profiling and other molecular testing across all major diseases.
By Nick Paul Taylor • April 18, 2025 -
Deep Dive
A judge blocked the FDA’s plan to regulate LDTs. What now?
One attorney said the ruling “isn’t quite the stake in the heart.”
By Susan Kelly • April 16, 2025 -

 The image by Coolcaesar is licensed under CC BY-SA 4.0
The image by Coolcaesar is licensed under CC BY-SA 4.0
Cepheid to lay off 167 people in California
The company laid off more than 1,300 people in California in 2023 and 2024 as respiratory sales fell from the peak hit during the pandemic.
By Nick Paul Taylor • April 14, 2025 -
Trump tariffs
China probes US-made CT tubes after Trump hikes tariffs
The investigation indicates that China will likely favor local medtech companies over foreign competition amid international economic tensions, a J.P. Morgan analyst said.
By Elise Reuter • Updated April 9, 2025 -
Labcorp launches blood test to accelerate Alzheimer’s diagnosis
Manufacturers of Alzheimer’s disease drugs have identified blood tests as a way to accelerate diagnosis and treatment.
By Nick Paul Taylor • April 7, 2025 -
Trump tariffs
Tariffs send healthcare industry into ‘unchartered waters’
Provider groups and health systems warn tariffs will test healthcare’s fragile supply chain. Wall Street, meanwhile, said medtech businesses will face pressure, particularly diabetes tech firms.
By Susanna Vogel , Susan Kelly • April 4, 2025 -
Texas judge overturns FDA’s lab developed test regulation, siding with industry groups
The judge vacated the FDA’s final rule, which was strongly opposed by the laboratory industry, and remanded the matter to HHS Secretary Robert F. Kennedy Jr.
By Susan Kelly • April 1, 2025 -
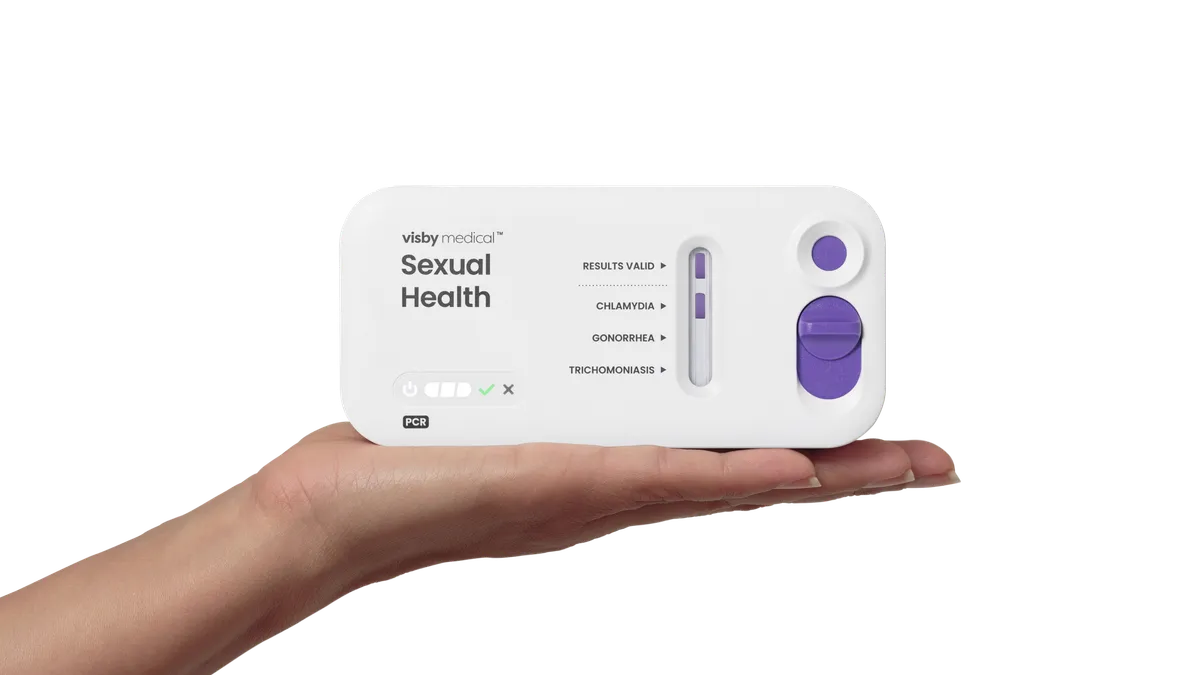
FDA OKs first at-home test for 3 STIs
Visby Medical’s at-home diagnostic, which can be purchased without a prescription, tests for chlamydia, gonorrhea and trichomoniasis.
By Nick Paul Taylor • March 31, 2025 -
23andMe files for bankruptcy; CEO Anne Wojcicki resigns
The DNA-testing company plans to sell its assets after the board rejected an acquisition proposal from Wojcicki.
By Elise Reuter • March 24, 2025