Diagnostics: Page 46
-

 "White House Press Briefing". Retrieved from The White House.
"White House Press Briefing". Retrieved from The White House.
As LabCorp, Quest feel COVID-19 strain, Trump admin pegs hopes on POC tests
Brett Giroir, the administration's lead for coronavirus diagnostics, said the goal is for rapid point-of-care tests from Abbott, BD and Quidel, performed outside of lab settings, to alleviate commercial testing capacity constraints.
By Greg Slabodkin • July 8, 2020 -
FDA warns of false positives with BD coronavirus diagnostic
On the heels of greenlighting BD's antigen test, FDA flagged an issue with its molecular test offering, telling healthcare professionals to treat positive results as “presumptive” and consider confirming findings via a different test.
By Nick Paul Taylor • July 7, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
BD launches coronavirus antigen test, 2nd to get FDA emergency use authorization
The rapid, point-of-care diagnostic joins Quidel's, which had been the only option in the category for nearly two months. Still, FDA notes antigen tests are more likely than molecular diagnostics to miss active coronavirus infections.
By Greg Slabodkin • July 6, 2020 -
CDC gets FDA nod for coronavirus-flu combination diagnostic
The emergency use authorization comes months after BioFire and Qiagen got backing for similar tests and as the coming flu season adds a new complexity to diagnosis.
By Nick Paul Taylor • July 6, 2020 -
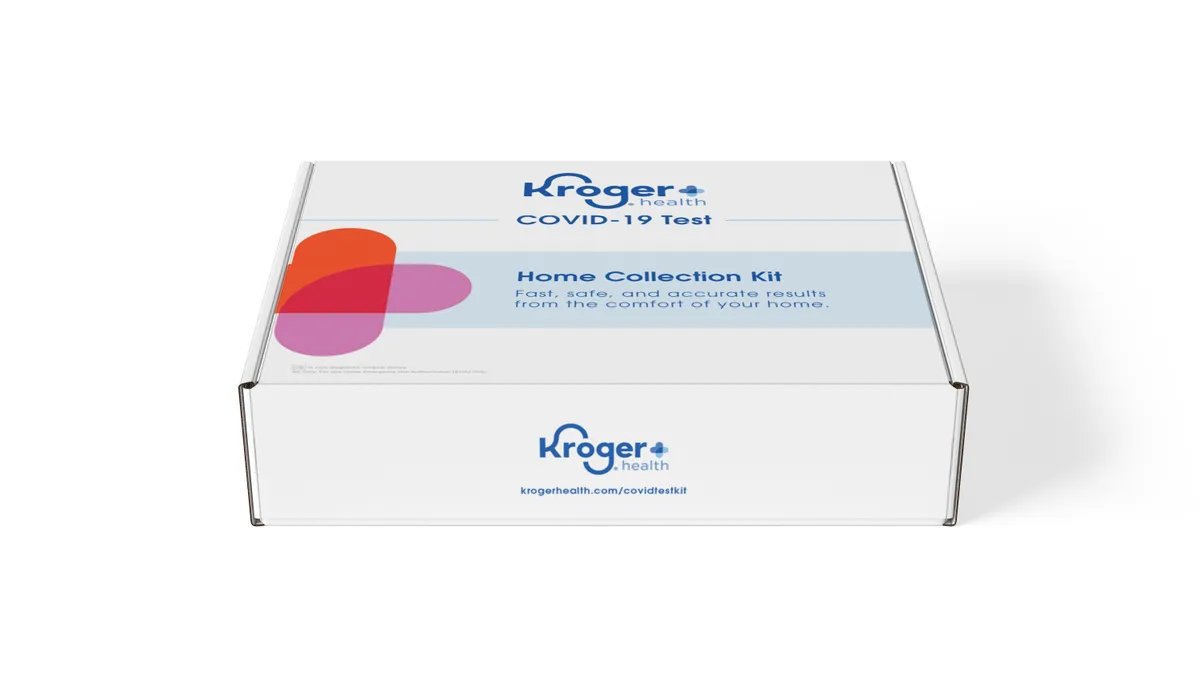
Kroger wins EUA for at-home coronavirus test kit
The grocer will make the home-collection kits available to employees this week and, along with partner Gravity Diagnostics, plans to process up to 60,000 tests weekly by the end of July.
By Jeff Wells • July 1, 2020 -
Notified body poll finds multi-month waits for COVID-19 conformity assessments
More than half surveyed by the European Commission expect to spend six months to a year processing applications for certain devices addressing coronavirus.
By Nick Paul Taylor • July 1, 2020 -
HHS 'expects to renew' COVID-19 emergency, spokesperson says
The department did not respond to requests for comment Tuesday morning. The public health emergency is currently set to expire at the end of July.
By Shannon Muchmore • June 30, 2020 -
As Beckman enters US COVID-19 antibody market, how do rivals compare?
Comparing the accuracy of the Danaher subsidiary's test to products from big rivals like Abbott, Roche and Siemens Healthineers depends on who's doing the assessment and when a sample is taken.
By Nick Paul Taylor • June 30, 2020 -
FDA nod to Boston Scientific cardiac monitor clears challenge to Abbott, Medtronic
The medtech giant is betting remote adjustment will put its LUX-Dx insertable system in a position to take on rivals, which have a years-long head start.
By Nick Paul Taylor • June 29, 2020 -

 Retrieved from Flickr.
Retrieved from Flickr.
Labs warn COVID-19 testing demand will soon top capacity as new hotspots emerge
With the spike in coronavirus cases in the South and West, commercial labs say they don't have the resources to keep up. Quest said in an update late Monday turnaround times for some molecular test results are now 3 to 5 days.
By Greg Slabodkin • Updated June 30, 2020 -
Payer coverage of employer, surveillance COVID-19 tests not required, feds say
As return-to-work offerings proliferate from lab and pharmacy giants like Quest, LabCorp and CVS, new federal guidance says health insurers aren't obligated to pay for the testing services.
By Maria Rachal • June 25, 2020 -

 "State Public Health Laboratory in Exton Tests for COVID-19" by Governor Tom Wolf is licensed under CC BY 2.0
"State Public Health Laboratory in Exton Tests for COVID-19" by Governor Tom Wolf is licensed under CC BY 2.0
UK study to validate, compare COVID-19 tests in real-world settings
The hospital arm will assess 10 to 20 new diagnostics the National Health Service has identified as high priority, including those designed to deliver results at the point of care in minutes, which could include Abbott’s ID NOW.
By Nick Paul Taylor • June 25, 2020 -
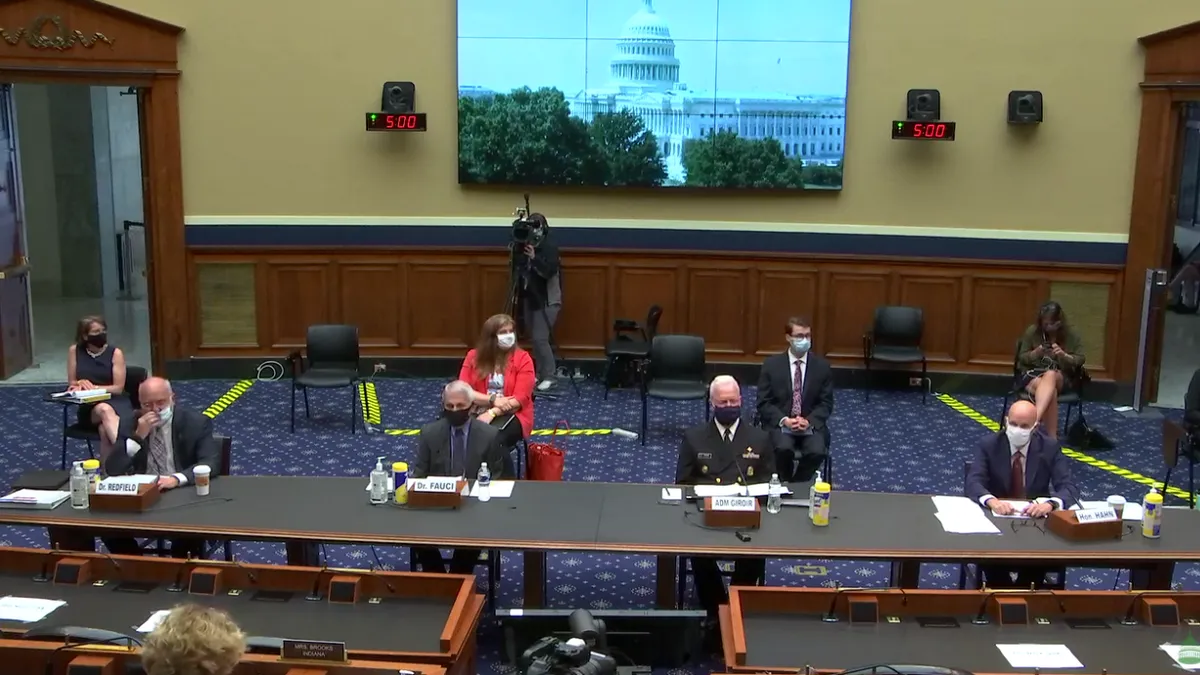
Fauci: Trump never asked to slow down testing, says US needs more, not less
During a House panel Tuesday, top U.S. infectious disease doctor Anthony Fauci and other public health officials denied they'd been directed to impede testing efforts as the White House targets up to 50 million tests a month by fall.
By Rebecca Pifer Parduhn • June 24, 2020 -
 "Florida National Guard" by The National Guard is licensed under CC BY 2.0
"Florida National Guard" by The National Guard is licensed under CC BY 2.0
5-minute, quantitative COVID-19 antibody test snags BARDA backing
MBio's offering could assess whether a patient has protective levels of antibodies and is a candidate to donate convalescent plasma, a potential treatment for critically ill COVID-19 patients.
By Susan Kelly • June 24, 2020 -
CVS launches COVID-19 return-to-work service with testing
Questions about testing accuracy and hesitancy by employers haven't stopped big healthcare companies, including Quest, LabCorp and UnitedHealth Group, from jumping into the space with new products.
By Rebecca Pifer Parduhn • June 24, 2020 -
New Guardant genomic database could be worth $2B, analysts say
The company said clinical information from repeat liquid biopsies in its 86,000-patient database will show how tumors evolve, information that may be attractive to biopharma partners.
By Nick Paul Taylor • June 24, 2020 -
Deep Dive
No silver bullet: LabCorp and Quest hawk return-to-work COVID-19 tests but employers not rushing in
One employer group official noted "hype" around antibody testing with inconclusive evidence about the immunity of individuals who test positive for the antibodies.
By Greg Slabodkin • June 22, 2020 -

 "Man in face mask in front of Union Station at 50 Massachusetts Avenue, NE, Washington DC on Thursday morning,19 March 2020" by Elvert Barnes Photography is licensed under CC BY-SA 2.0
"Man in face mask in front of Union Station at 50 Massachusetts Avenue, NE, Washington DC on Thursday morning,19 March 2020" by Elvert Barnes Photography is licensed under CC BY-SA 2.0
BARDA backs study of Empatica's COVID-19 early warning platform
The system, which measures physiological data including blood volume pulse and electrodermal activity, is the latest in a growing list of wearables being primed to detect and monitor infections.
By Susan Kelly • June 22, 2020 -
Trump admin sitting on $14B for testing, tracing, Democratic senators say
A letter urging the administration to ramp up its COVID-19 spending comes a day after President Donald Trump told a rally he had directed officials to slow down testing to keep case numbers low.
By Rebecca Pifer Parduhn • June 22, 2020 -
Invitae cash-and-stock buyout of ArcherDX a done deal
The medical genetic testing company swept in after the assay developer announced plans to go public. Invitae will now offer tests for more stages of cancer care, from assessing disease risk to monitoring its progression.
By Maria Rachal • Updated Oct. 5, 2020 -

 Dufour, Tia. (2020). "White House Press Briefing" [Photograph]. Retrieved from Flickr.
Dufour, Tia. (2020). "White House Press Briefing" [Photograph]. Retrieved from Flickr.
Pandemic pushes FDA to 'accelerate' real-world evidence efforts, Hahn says
The agency has grappled with how to leverage real-world data in regulating medical devices, and is now leaning on it to update emergency use authorizations, FDA chief Stephen Hahn said at a forum on Thursday.
By Maria Rachal • June 19, 2020 -
FDA cracks down on sellers of at-home COVID-19 antibody tests
A series of warning letters is the latest example of U.S. regulators weeding out serology tests that don't meet the agency's standards.
By Nick Paul Taylor • June 18, 2020 -
TÜV SÜD becomes 4th notified body designated under IVDR
It's the first notified body OK'd by the European Commission in 2020 to increase capacity for the forthcoming In Vitro Diagnostic Regulation, set to come into force in less than two years.
By Nick Paul Taylor • June 18, 2020 -
FDA revokes emergency authorization for Chembio's COVID-19 antibody test
It's the first time the agency has exercised such authority for a coronavirus test, citing more false results than expected. Chembio Diagnostics' stock plunged 60% Wednesday.
By Greg Slabodkin • June 17, 2020 -
NIH loops Quest into effort to test 10K Americans for antibodies against coronavirus
The agency did not disclose which antibody test it will use as part of the All of Us initiative, saying only that the assay has FDA emergency use authorization and is designed to detect IgG antibodies.
By Nick Paul Taylor • June 17, 2020