Diagnostics: Page 63
-
ACLA praises CMS for boosting data collection
The clinical lab trade group lauded the agency for taking steps to increase the number of laboratories submitting data informing Medicare payment rates.
By David Lim • Nov. 5, 2018 -
FDA warns about Roche test after reports of patient strokes
Two patients suffered serious injuries involving strokes, prompting a warning that inaccurate results from the Swiss pharma's CoaguChek could cause serious injuries or death when taken with the blood thinner warfarin.
By Nick Paul Taylor • Nov. 2, 2018 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
23andMe wins FDA clearance for first-of-its-kind genetic test
The agency also sent a safety communication warning about the danger of relying on genetic tests that have not been reviewed by FDA making claims about patient response to individual medications.
By David Lim , Nick Paul Taylor • Nov. 1, 2018 -
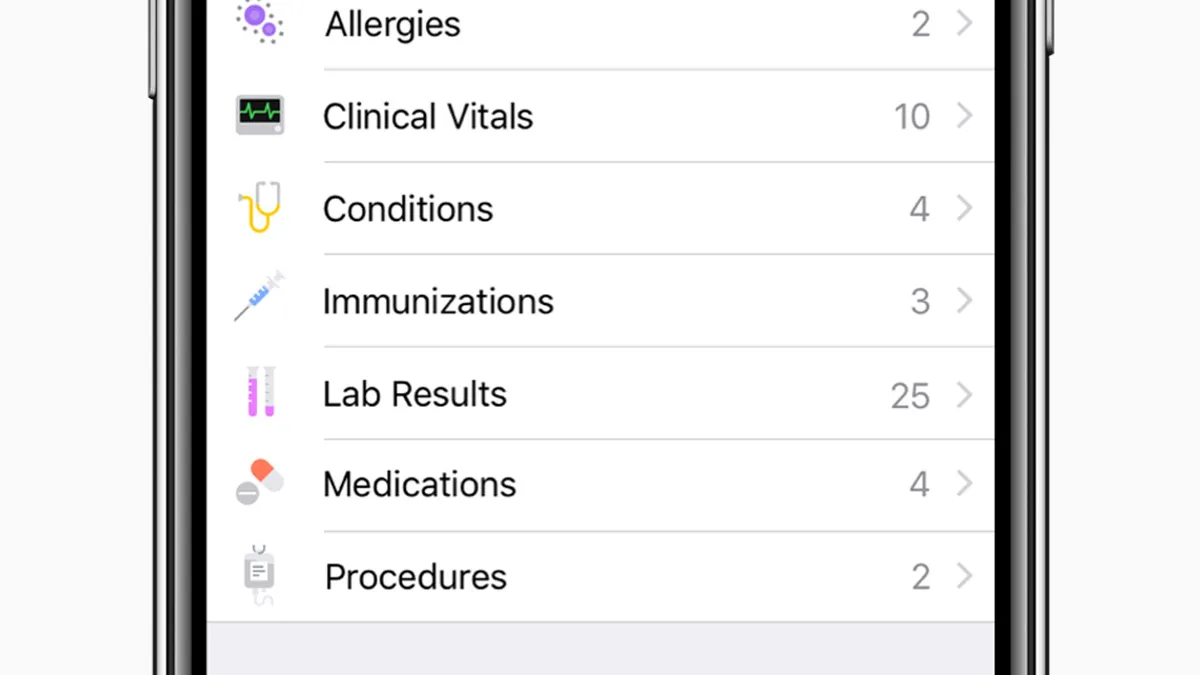
 Retrieved from Apple on January 24, 2018
Retrieved from Apple on January 24, 2018
LabCorp rolls out Apple Health integration for lab test results
CEO David King argued the move to enable patients to view their testing results comes at a time when individuals are increasingly interested in gaining a "more holistic view of their health."
By David Lim • Oct. 31, 2018 -
Deep Dive
5 sticking points in the EU medical device reg transition
Brexit and notified body capacity are among the uncertainties, as many in the medtech sector are uninformed.
By Meg Bryant • Oct. 29, 2018 -
Philips launches all-in-one breast ultrasound product
The technology package is designed to make exams easier and faster for patients and physicians.
By Susan Kelly • Oct. 29, 2018 -
FDA clears Ansh Labs menopausal status test
Physicians can use the results to inform actions to prevent osteoporosis and heart disease.
By Nick Paul Taylor • Oct. 25, 2018 -
FDA starts 510(k) pilot to cut optical tomography processing times
The program aims to give developers of optical coherence tomography devices the chance to discuss premarket performance testing recommendations with the agency.
By Nick Paul Taylor • Oct. 24, 2018 -
Quest disappoints with full year revenue forecast cut
The lab giant said its business remains pressured by an industrywide decline in hepatitis C testing. Investors sent shares down 7% in morning trade.
By Susan Kelly • Updated Oct. 23, 2018 -
Myriad's BRCA test supports successful ovarian cancer trial
The molecular diagnostics company plans to file to expand use of the genetic test.
By Nick Paul Taylor • Oct. 23, 2018 -
Abbott heart attack test shows early promise in small study
The diagnostic was as successful at ruling out acute myocardial infarction as a lab-based assay.
By Nick Paul Taylor • Oct. 18, 2018 -
Abbott medical device, diagnostic units drive Q3 growth
CEO Miles White said Abbott plans to submit its data to FDA in the coming weeks to back an expanded indication for MitraClip.
By David Lim • Oct. 17, 2018 -
FDA clears Pfizer PARP drug, Myriad companion test for breast cancer
Patients must be selected for treatment based on Myriad's test to identify those eligible for talazoparib.
By Susan Kelly • Oct. 17, 2018 -
Immunodiagnostic demand powers Roche Q3 results
The 6% sales growth at the diagnostic business extends the company’s streak of positive quarters.
By Nick Paul Taylor • Oct. 17, 2018 -
FDA OKs DNA-based test for blood transfusion compatibility
Grifols unit Progenika Biopharma's product is the second molecular assay approved for use in transfusion medicine and the first approved to report genotypes as final results.
By Susan Kelly • Oct. 12, 2018 -
Framework aims to employ e-triggers to improve diagnostic safety
Authors writing in BMJ Quality and Safety offer tools they say would not only detect potential diagnostic events and monitor error rates but also help to identify patients at greater risk of future adverse events.
By Meg Bryant • Oct. 10, 2018 -
Prostate cancer imaging agent meets one of two study endpoints
The results mark Progenics Pharmaceuticals' second clinical trial for a prostate cancer imaging agent to show mixed results in as many months.
By Susan Kelly • Oct. 8, 2018 -
Karius posts clinical data on heart infection test
The test identified the pathogen affecting one of the three patients failed by traditional methods.
By Nick Paul Taylor • Oct. 5, 2018 -
UK recalls 58K pregnancy tests after false positive reports
The digital displays of some devices erroneously told users they were pregnant.
By Nick Paul Taylor • Oct. 5, 2018 -
European Parliament passes device-friendly changes to draft HTA law
The amendments will limit the types of devices that undergo joint clinical assessments.
By Nick Paul Taylor • Oct. 4, 2018 -
Regulatory professionals working in devices paid far less than pharma peers
Reg managers in the drug industry take home around 75% more than their device counterparts.
By Nick Paul Taylor • Oct. 4, 2018 -
Ready or not, medtech braces for new EU landscape
Companies should not assume they'll get extra time to adapt, warned Vicki Anastasi, VP for medical device and diagnostics research at ICON.
By Kim Dixon • Oct. 1, 2018 -
FDA clears NGS test to detect residual blood cancer cells
Adaptive Biotechnologies' assay detects and monitors minimal residual disease in multiple myeloma and B-cell acute lymphoblastic leukemia patients.
By Susan Kelly • Oct. 1, 2018 -
New IMDRF committee to tackle cybersecurity threats
The FDA device chief laid out takeaways from the group's Beijing meeting.
By Kim Dixon • Sept. 27, 2018 -
Global device industry set to grow 5.6% a year through 2024: report
In vitro diagnostics is projected to remain as the No. 1 specialty in 2024 with annual sales of $79.6 billion and a 13.4% share of the medical device industry.
By Susan Kelly • Sept. 26, 2018



