Digital Health
-
Senseonics launches automated insulin dosing system with 1-year CGM
The company has integrated its Eversense 365 implant with Sequel Med Tech’s insulin delivery system to automate the management of Type 1 diabetes.
By Nick Paul Taylor • Feb. 20, 2026 -
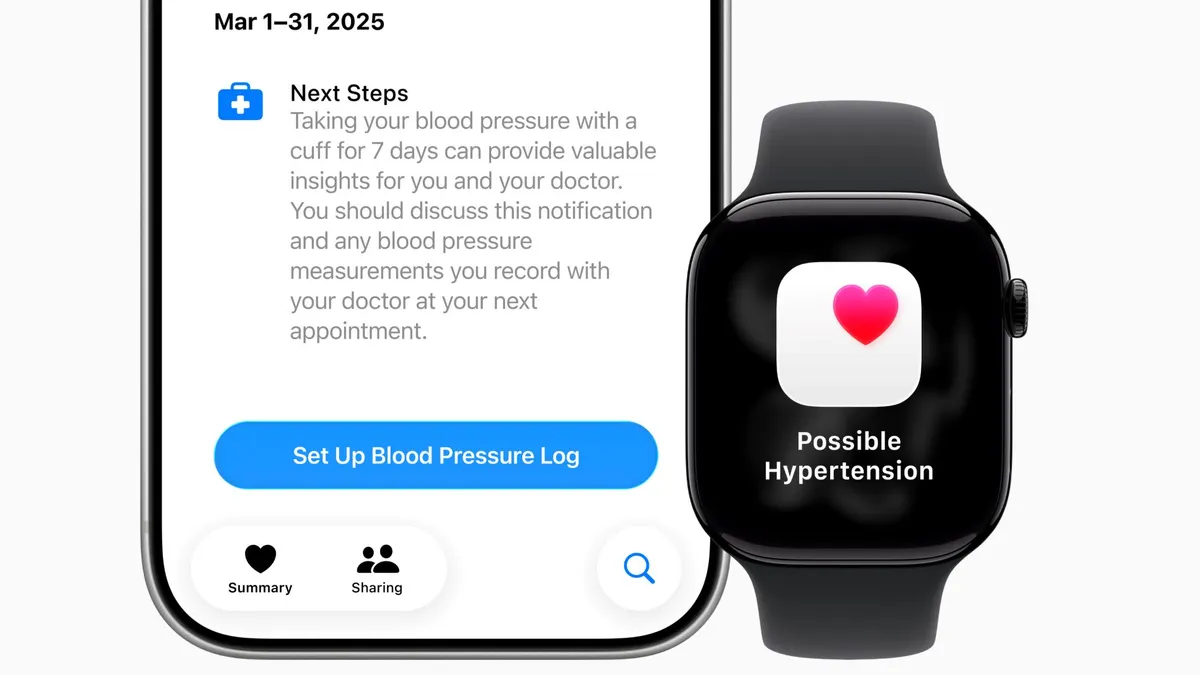
Apple Watch’s hypertension feature may miss some cases, researchers find
The absence of an alert provides limited reassurance, particularly for older adults or people with an elevated risk of high blood pressure, according to the study’s authors.
By Elise Reuter • Feb. 19, 2026 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
FDA guidance eases wearables oversight. But experts have questions about what’s next.
Experts say two new guidances — issued without the usual public comment period — leave questions about how patients will navigate a growing pool of wearables.
By Elise Reuter • Jan. 27, 2026 -
AdvaMed leaders on tariffs, MDUFA and wearables
AdvaMed CEO Scott Whitaker and Mick Farrell, new board chair of the trade group, weighed in on important issues for the medtech industry one year into the Trump administration.
By Elise Reuter • Jan. 22, 2026 -
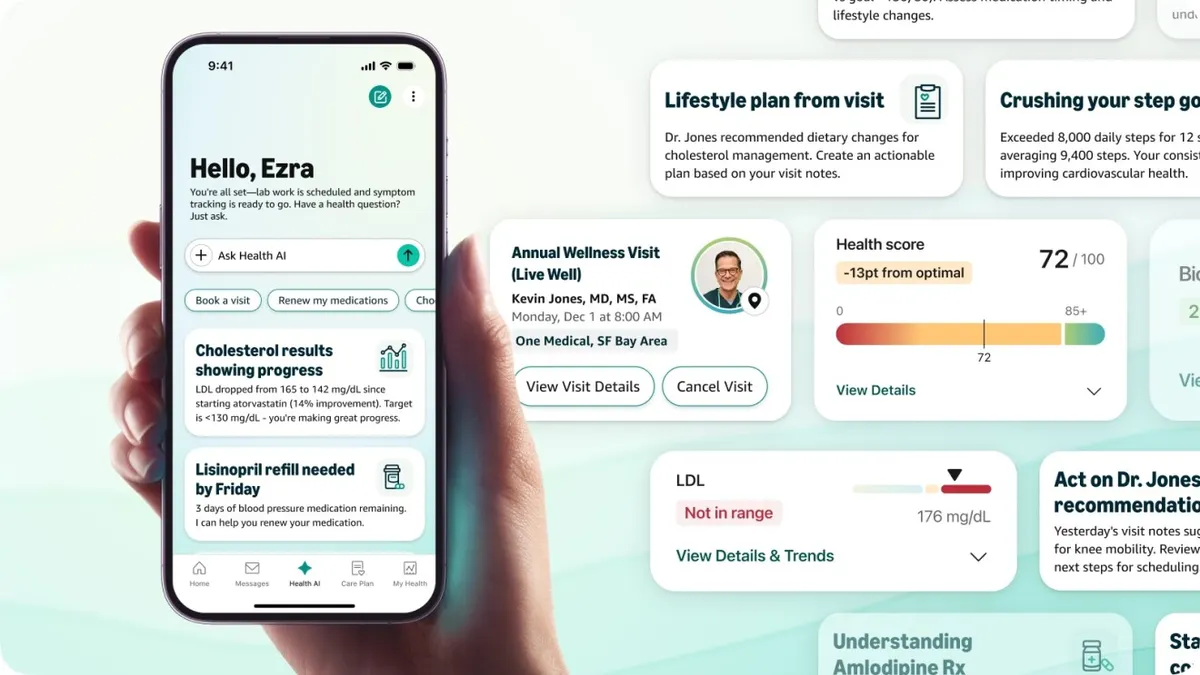
Amazon launches health AI chatbot for One Medical members
The assistant, which can answer health questions and help schedule appointments, comes on the heels of other health-focused AI tools from OpenAI and Anthropic.
By Emily Olsen • Jan. 22, 2026 -
ECRI names misuse of AI chatbots as top health tech hazard for 2026
The nonprofit said technologies like ChatGPT have suggested incorrect diagnoses, invented body parts and otherwise provided information that could lead to harm.
By Nick Paul Taylor • Jan. 22, 2026 -
Digital health funding increases in 2025, spurred by AI: report
Startups raised $14.2 billion last year, the highest funding total since 2022. Health AI companies collected 54% of total funding, according to Rock Health.
By Emily Olsen • Jan. 15, 2026 -
FDA exempts more wearable, AI features from oversight
In a pair of guidance documents released Tuesday, the regulator clarified the types of wellness features and clinical decision support tools that don’t fall under medical device oversight.
By Elise Reuter • Jan. 8, 2026 -
Q&A
Ōura’s Ricky Bloomfield proposes new regulatory pathway for wearable health features
The chief medical officer is interested in an easier path to market for health screening features in wearable devices.
By Elise Reuter • Jan. 5, 2026 -
FDA gets mixed feedback on performance monitoring for AI
Medtech industry groups said the FDA should use existing regulatory and quality tools to monitor performance, while medical groups said device manufacturers should be responsible for monitoring AI.
By Elise Reuter • Dec. 16, 2025 -
FDA pilots allowing digital health devices access to CMS payment program
Companies can ask the FDA to waive premarket authorization and investigational device requirements while they collect real-world data in a CMS program.
By Nick Paul Taylor • Dec. 10, 2025 -
Deep Dive
What medtech firms can learn from Whoop’s warning letter
An FDA warning letter about the wearable company’s blood pressure feature raises questions about the blurring lines between medtech and wellness.
By Elise Reuter • Dec. 8, 2025 -
Ceribell wins FDA clearance for seizure-detection algorithm in neonates
By acquiring new accounts and expanding into NICUs managed by existing clients, Ceribell calculates it can unlock a $400 million market.
By Nick Paul Taylor • Nov. 25, 2025 -
Health tech venture capital investment rebounds in 2025: report
Funding through the third quarter surpassed last year’s total, according to PitchBook. Meanwhile, average deal sizes are skyrocketing as high valuations — especially for AI startups — drive larger rounds.
By Emily Olsen • Nov. 24, 2025 -
Jury orders Apple to pay Masimo $634M in patent fight
Apple said it plans to appeal the verdict, which could delay Masimo’s collection of the damages by as much as two years, one analyst said.
By Susan Kelly • Nov. 17, 2025 -
Synchron raises $200M to prepare for brain computer interface launch
The company will use the money to accelerate pivotal trials and prepare for commercial launch of the Stentrode BCI system.
By Nick Paul Taylor • Nov. 7, 2025 -
J&J awards first grants from AI surgery fund
Mayo Clinic and QAS.AI will receive funding and mentorship to help advance their projects.
By Elise Reuter • Oct. 27, 2025 -
Profile
Hemorrhage can be a serious problem after pregnancy. Here’s how one medtech founder is tackling it
Kelsey Mayo, CEO of Armor Medical, received the grand prize in MedTech Innovator’s pitch competition for a wearable technology to detect serious blood loss early.
By Elise Reuter • Oct. 23, 2025 -
Ōura to pursue FDA clearance of blood pressure feature for smart ring
The company has received approval to validate and define the feature in a study that is scheduled to start this year.
By Nick Paul Taylor • Oct. 20, 2025 -
Ōura raises $900M to develop new health features
The investment values the smart-ring maker at $11 billion.
By Elise Reuter • Oct. 17, 2025 -
5 AI takeaways from AdvaMed’s conference
Medical device firms discussed privacy, regulations and prioritizing projects as AI becomes more prevalent in the industry.
By Elise Reuter • Oct. 14, 2025 -
Digital health funding outpacing last year as huge rounds increase: report
Investment in 2025 has reached $9.9 billion, exceeding the $8.4 billion that was raised through the third quarter last year, according to Rock Health.
By Emily Olsen • Oct. 9, 2025 -
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
By Nick Paul Taylor • Oct. 1, 2025 -
Medtronic expands AI, robotics hub in London; Virtuoso robot gets FDA breakthrough status
The Medtronic center will facilitate surgeon collaboration and AI-powered decision support. At Virtuoso Surgical, a robotic approach to bladder lesion removal is expected to improve precision.
By Susan Kelly • Sept. 24, 2025 -
Cardiosense names Eric Meizlish as CEO
Cardiosense’s switch to a leader with experience scaling healthtech companies comes as it works to build on the recent 510(k) clearance of CardioTag.
By Nick Paul Taylor • Updated Sept. 25, 2025