Digital Health: Page 12
-
Stanford team reports progress with Theranos-like finger prick blood test
The researchers have created a system for capturing and analyzing thousands of molecules from a single drop of blood.
By Nick Paul Taylor • Jan. 23, 2023 -
Opinion
Realizing the promise of AI for medical imaging and diagnosis
Alissa Hsu Lynch, global lead for MedTech Strategy & Solutions at Google Cloud, explains how medtech companies can benefit from applying AI to the explosion of medical imaging data.
By Alissa Hsu Lynch, global lead for MedTech Strategy & Solutions at Google Cloud • Jan. 19, 2023 -
Bayer to buy AI imaging platform Blackford Analysis to bolster radiology growth strategy
Bayer aims to outpace the broader radiology segment by investing in fast-growing technologies such as imaging AI.
By Elise Reuter • Updated Jan. 18, 2023 -
Akili cuts 30% of staff to focus cash on growing prescription video game sales
The move comes as the company seeks a path forward after sales of $82,000 in the third quarter. Over the last five months, its share price has fallen 90%.
By Nick Paul Taylor • Jan. 13, 2023 -

 Courtesy of Press release https://verily.com/blog/verily-expands-stephen-gillett-s-role-to-president-and-chief-operating-officer/
Courtesy of Press release https://verily.com/blog/verily-expands-stephen-gillett-s-role-to-president-and-chief-operating-officer/
Google sibling Verily to lay off about 200 workers as it focuses on precision risk business
The company, which has more than 1,600 employees, will stop work on some products including its healthcare analytics software program.
By Nick Paul Taylor • Jan. 12, 2023 -
Apple Watch infringed on Masimo patent, judge rules, raising prospect of import ban
Masimo’s CEO said the ruling “exposes Apple as a company that takes other companies’ innovations and repackages them.”
By Nick Paul Taylor • Updated Jan. 12, 2023 -
Beta Bionics hires Harvard diabetes expert as CMO ahead of artificial pancreas launch
Steven Russell has joined the company as chief medical officer, completing a new leadership team that began taking shape with the appointment of Sean Saint as CEO last year.
By Nick Paul Taylor • Jan. 10, 2023 -
Cardiac wearable maker iRhythm sees ‘nice progress’ in recovering from Q3 challenges
The company reported more than 20% growth in patient registration and improvement in its returned device rate.
By Nick Paul Taylor • Jan. 10, 2023 -
Apple infringed on AliveCor wearable patents, ITC says in final ruling; import ban suspended
The commission found that Apple has infringed on two AliveCor patents and thereby violated a law that can trigger an import ban.
By Nick Paul Taylor • Jan. 4, 2023 -
Olympus agrees to $80M takeover of artificial intelligence endoscopy startup Odin Vision
The acquisition gives Olympus control of cloud-based AI systems to help detect and characterize cancerous and precancerous tissues during colonoscopies and gastroscopies.
By Nick Paul Taylor • Dec. 21, 2022 -
As diabetes technology advances, blood glucose control declines: study
Mexican-American and non-Hispanic Black adults saw the greatest drop as the proportion of patients with glycemic control fell from 29.2% between 1988 and 1994 to 27.5% between 2013 and 2020.
By Nick Paul Taylor • Dec. 21, 2022 -
Novo Nordisk expands Amalgam Rx insulin app pact, plots integration with other devices
Supported by Novo Nordisk, Amalgam Rx will incorporate and integrate with connected insulin delivery devices, continuous glucose monitors and electronic health records.
By Nick Paul Taylor • Dec. 20, 2022 -
Click wins FDA breakthrough designation for prescription digital migraine therapy
The designation would give Click Therapeutics expedited review of its preventive treatment for episodic migraine in adults.
By Nick Paul Taylor • Dec. 19, 2022 -
Pfizer to partner with Mayo Clinic spinoff on early cardiac amyloidosis detection
Anumana and Pfizer will build an algorithm using ECG data to identify patients who may be at risk of developing the serious heart condition.
By Elise Reuter • Dec. 16, 2022 -
Masimo links passive health monitors to home stereos amid legal row with Apple
The move integrates the consumer audio brands Masimo bought in April with its wearable health monitors.
By Peter Green • Dec. 15, 2022 -
Deep Dive
Apple locked in court battles with small medtech firms over allegations of stolen technology
AliveCor and Masimo are looking to bar Apple from selling watches that use technology the firms claim they invented.
By Elise Reuter , Nick Paul Taylor • Updated Dec. 13, 2022 -
FDA outlines benefits and risks of emerging augmented and virtual reality medical devices
AR/VR may increase access to healthcare and make procedures less invasive, but could potentially cause cybersickness and head and neck strain.
By Nick Paul Taylor • Dec. 8, 2022 -
Apple prevails in AliveCor patent dispute ahead of potential Watch import ban
Patent board ruled that all 30 claims are unpatentable because they are obvious in view of other patents.
By Nick Paul Taylor • Dec. 8, 2022 -
Stanford’s smart bandages accelerate wound healing in preclinical tests
The bandages deliver electrical stimulation to accelerate wound healing in mice by 25% compared to standard sterile dressings.
By Nick Paul Taylor • Dec. 7, 2022 -
AI detects prediabetes in CGM data, opening up new use for device: study
By correctly identifying many patients with prediabetes or Type 2 diabetes, researchers say CGMs can be used to prevent the disease.
By Nick Paul Taylor • Dec. 6, 2022 -
The 10 biggest medtech stories of 2022
MedTech Dive reporting this year has explored how companies maintained momentum even amid supply shortages and rising inflation rates.
By MedTech Dive staff • Dec. 3, 2022 -
Eargo raises $32M to fight rivals in over-the-counter hearing aid market
Investment firm takes majority stake, as Eargo faces competition from new rivals including Sony and Bose-partnered Lexie.
By Nick Paul Taylor • Dec. 1, 2022 -
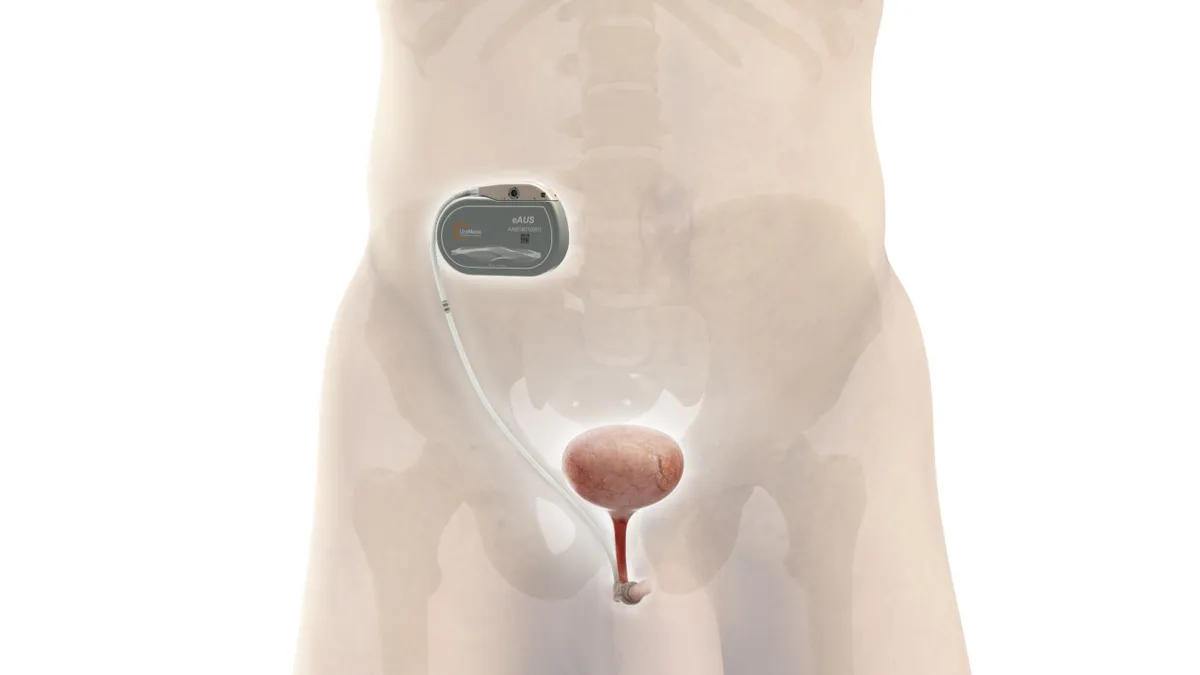
UroMems starts clinical trial of smart, automated implant to treat incontinence
The device is designed to improve on products such as Boston Scientific’s AMS 800 by eliminating the need for manual adjustments.
By Nick Paul Taylor • Nov. 30, 2022 -
GE Healthcare, MediView XR partner to launch augmented reality X-ray imaging system
The companies are pitching the product as a response to physician demand for streamlined workflows and improved ergonomics.
By Nick Paul Taylor • Nov. 30, 2022 -
Google Health licenses breast cancer detection AI to iCAD
Applying Google’s AI to breast-cancer screening algorithms may alleviate a shortage of trained physicians and enable faster tumor treatment.
By Nick Paul Taylor • Nov. 29, 2022