Digital Health: Page 28
-

 Retrieved from Akili Interactive/Businesswire on June 15, 2020
Retrieved from Akili Interactive/Businesswire on June 15, 2020
In FDA 1st, game-based therapeutic gets marketing OK
Digital therapeutics developer Akili Interactive Labs won De Novo authorization for a treatment meant to improve attention function in children with ADHD, building on a rollout it began under a special COVID-19 policy.
By Maria Rachal • June 16, 2020 -
Heart rhythm bodies see 'clear concerns' with using wearables to detect arrhythmias
A paper published on Monday by the Heart Rhythm Society, along with counterpart groups in Asia, Europe and Latin America, found wearable-triggered false positives can cause unwarranted concerns and screening.
By Nick Paul Taylor • June 16, 2020 -
Siemens Healthineers, Geisinger ink 10-year digital health partnership
It's one of the medtech's largest partnerships of its kind in North America, as it plans to provide diagnostic imaging and artificial intelligence-enabled applications to the health system over the next decade.
By Greg Slabodkin • June 9, 2020 -
BARDA, Evidation team on early detection of COVID-19 using wearables
As part of an agency program to identify people infected with the virus, the company will analyze sleep and activity data, plus self-reported symptoms, aimed at creating an early warning algorithm.
By Nick Paul Taylor • June 5, 2020 -
Pandemic causes expected wearable shipments to plummet by 27M this year
But analysts expect a drawn-out recovery for the sector as wearables bolster their advanced health monitoring features and consumers remain increasingly health-conscious coming out of the COVID-19 crisis.
By Rebecca Pifer Parduhn • June 4, 2020 -
Emergency authorization granted to COVID-19 ICU prediction software
The FDA nod for CLEW Medical's system is among a series of regulatory OKs for products designed to inform care for infected patients.
By Nick Paul Taylor • May 28, 2020 -
Sponsored by Alexander Group
How leading commercial organizations will ramp upon re-open
Increased agility helps healthcare commercial leaders adapt to a new buyer journey.
May 27, 2020 -
ResMed, Medtronic embrace remote tech amid COVID-19, say it's here to stay
The crisis has sped adoption of digital health technologies to help hospitals create safe physical distances between healthcare workers and patients. Execs say some could become permanent products.
By Greg Slabodkin • May 26, 2020 -
Philips lung ultrasound, Beckman sepsis diagnostic receive BARDA coronavirus backing
The separate projects aim to incorporate machine learning algorithms to enhance technologies meant to support COVID-19 patients.
By Susan Kelly • May 19, 2020 -
UnitedHealth, Microsoft launch COVID-19 screening app for employers
The healthcare behemoth said it will control employees' medical data and manage opt-in and consent requirements for users. The app will not provide tracking or contact tracing information.
By Rebecca Pifer Parduhn • May 15, 2020 -
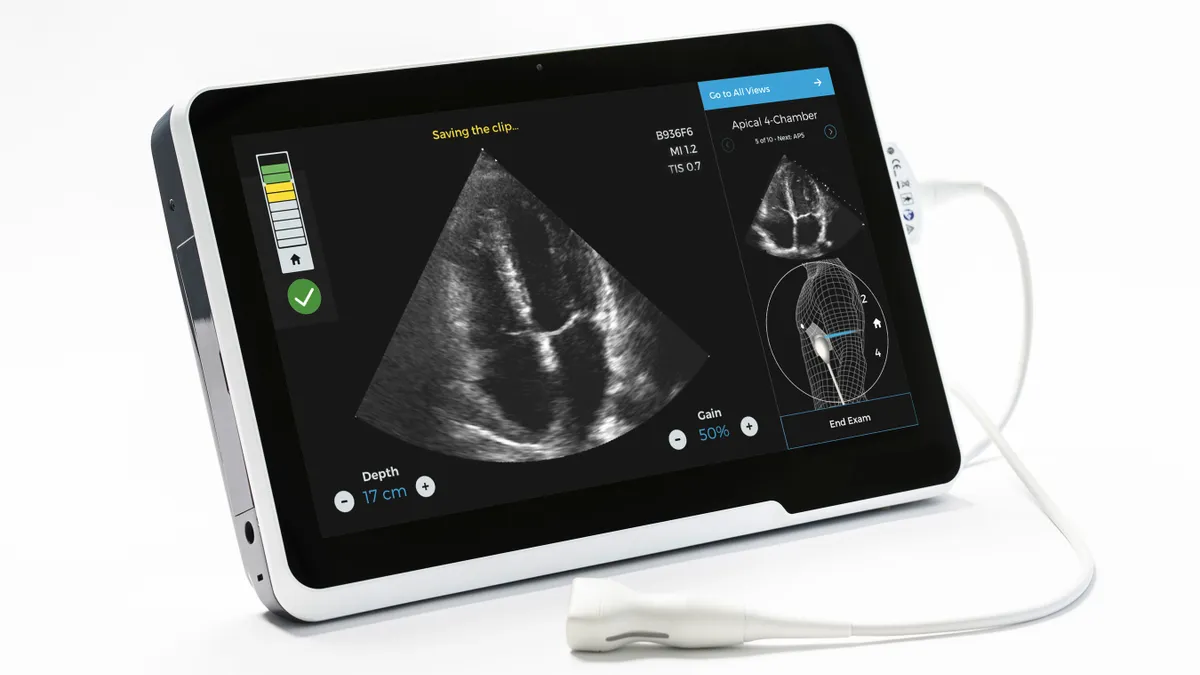
Philips, startups look to deploy new ultrasound tech amid coronavirus
The need for point-of-care imaging for lung and cardiac complications among COVID-19 patients has led to a flurry of expedited FDA reviews.
By Nick Paul Taylor • May 14, 2020 -
Eko algorithm gets FDA nod for cardiac screening of COVID-19 patients
Developed with the Mayo Clinic, Eko's algorithm gained breakthrough device status in December and review was further accelerated given its potential to identify patients at higher risk of poor outcomes with the coronavirus.
By Nick Paul Taylor • May 13, 2020 -
Deep Dive
Telehealth is having a moment. What does its future look like after COVID-19?
Employers should expect workers to embrace telehealth post pandemic, sources told HR Dive, even if concerns about billing and security persist.
By Ryan Golden • May 7, 2020 -
Fitbit launches its 1st large-scale, virtual AFib study
The atrial fibrillation detection effort follows work by wearables rival Apple targeting the stroke risk factor that affects 33.5 million people globally.
By Greg Slabodkin • May 6, 2020 -
COVID-19 spurs 'incredible demand' for ventilators, buoying ResMed's 16% revenue uptick
CEO Mick Farrell told investors sales of breathing assist machines added $35 million in coronavirus-related revenue. However, sleep testing declined by double digits.
By Greg Slabodkin • May 1, 2020 -
FDA encourages remote review of digital pathology slides amid COVID-19
The agency's latest effort to support greater access to medical devices during the pandemic follows a move by CMS that opened the door for pathologists to work away from the lab.
By Susan Kelly • April 27, 2020 -
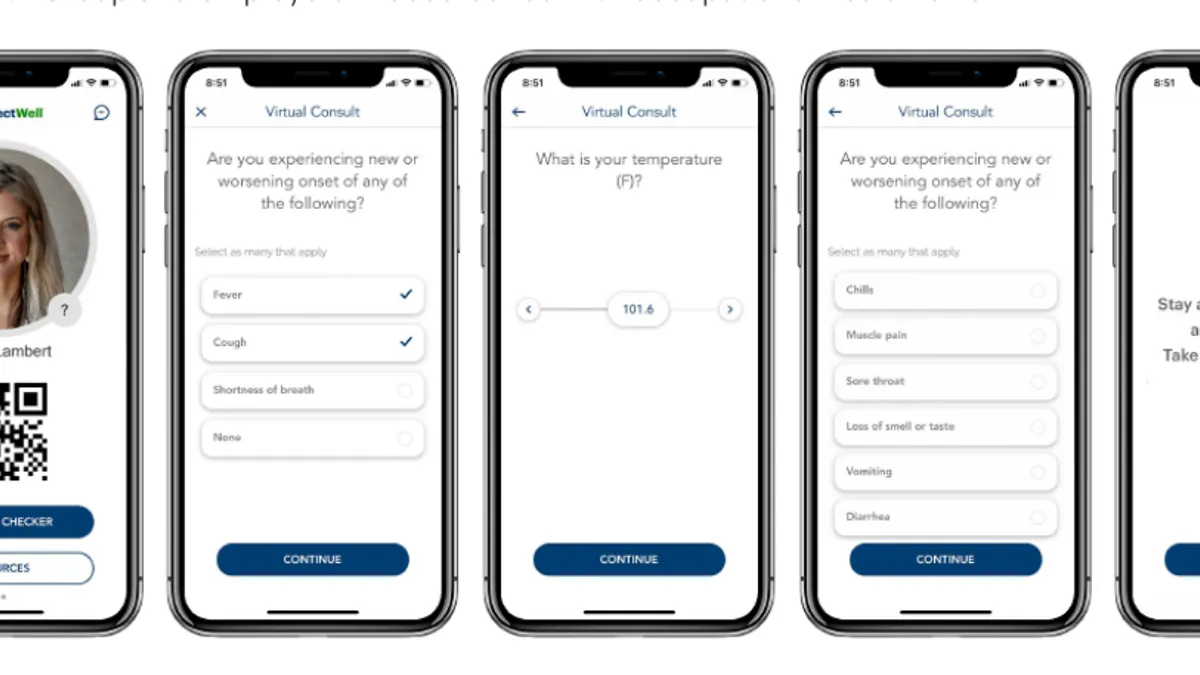
Digital therapeutic makers see opportunity as pandemic prompts FDA to ease rules
Akili Interactive Labs is rolling out its video game treatment for ADHD for the first time, while Pear Therapeutics weighs how products still in its pipeline may apply during the coronavirus crisis.
By Maria Rachal • April 24, 2020 -
Harvard, INSEAD authors pitch systemic regulatory approach to AI/ML devices
A new paper in Nature's digital medicine journal argues that real-world performance of specific SaMD products using artificial intelligence will vary considerably by hospital and other factors.
By Nick Paul Taylor • April 8, 2020 -
Digital health rakes in record $3.1B in Q1, but coronavirus slump may loom
"Though some of the economic blow will be tempered by demand for connected healthcare, we do not anticipate investment activity to keep pace with Q1 levels in the coming months," a new Rock Health report says.
By Rebecca Pifer Parduhn • April 7, 2020 -
FDA clears Sectra's digital pathology module for primary diagnostics as US market heats up
The clearance will intensify competition in the space initially monopolized by Philips, with big medtechs like Roche aiming to enter. The ability to review images from anywhere may be critical amid the coronavirus pandemic.
By Nick Paul Taylor • April 2, 2020 -
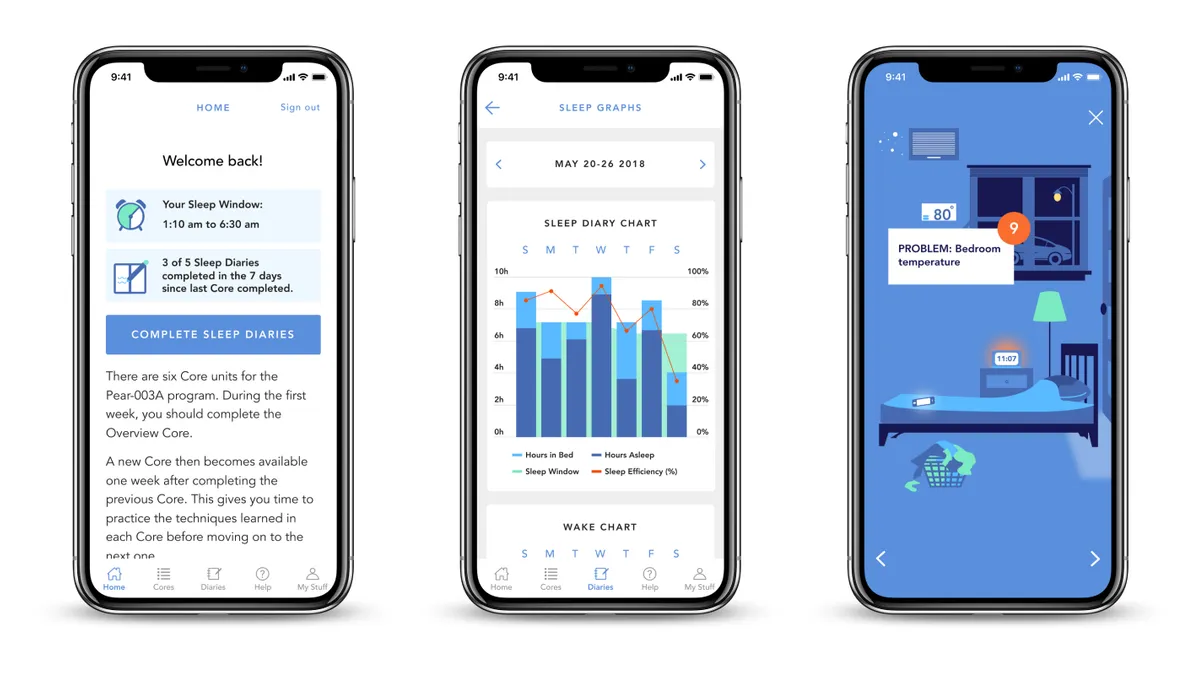
In Pre-Cert 1st, FDA clears app-based treatment for chronic insomnia
Pear Therapeutics, one of nine initial participants in an FDA pilot that rethinks software regulation, touted the product as the first to be submitted through the 510(k) pathway while also reviewed as part of Pre-Cert.
By Maria Rachal • March 27, 2020 -
Digital health advice from FDA amid coronavirus leaves more questions than answers
While the agency doesn't consider most apps and software systems for COVID-19 public health surveillance and communication to be regulated medical devices, the scope of regulation remains murky.
By Greg Slabodkin • March 27, 2020 -
FDA still trying to fine-tune Pre-Cert as pilot enters 2020
The Pre-Cert pilot, which includes Apple, J&J and Fitbit, is moving forward with the agency "working through the nine companies, trying to figure out how they actually do business," FDA's Bakul Patel told MedTech Dive.
By Greg Slabodkin • March 25, 2020 -
CDRH pushes out response due date extensions, teleconference policy by at least a month
Less than two weeks after FDA wrote to industry extending deadlines for premarket application responses and warning of disruptions to in-person meetings, the agency lengthened those coronavirus-era changes.
By Maria Rachal • Updated April 6, 2020 -
Disease management firms set to capitalize on Trump admin interoperability push
A key detail of a rule finalized last week could make it simpler for Omada Health, Propeller Health and Livongo to interface with large health system clients.
By Rebecca Pifer Parduhn • March 17, 2020