FDA: Page 95
-
Grassley questions FDA about 'troubling' cybersecurity audit
"Medical devices could be exploited by those same foreign actors to not only interfere with normal device operation, which could cause harm to patients, but also to steal personal medical information," the senator wrote.
By Nick Paul Taylor • Nov. 13, 2018 -
Deep Dive
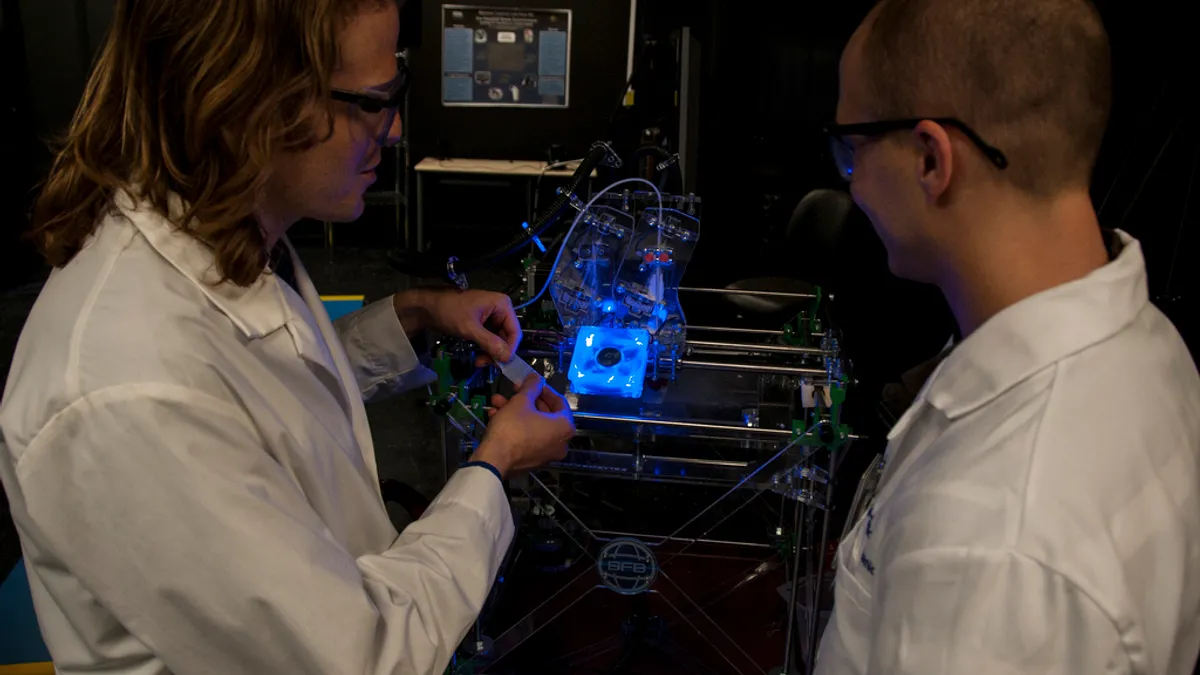
3D printing poised to disrupt healthcare
Health industry types are "100% convinced, and rightly so, that [3D printing] will be a core part of their business in five to 10 years," ISG's Michael Harmon said.
By Meg Bryant • Nov. 12, 2018 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA clears battery-powered Ebola test for emergency use
The regulatory decision adds to the testing capabilities of public health clinics and treatment centers.
By Nick Paul Taylor • Nov. 12, 2018 -
Monteris modifies brain ablation device to counter safety risk
FDA cleared a new model that features a fiber optic probe designed to stop the original heating problem.
By Nick Paul Taylor • Nov. 9, 2018 -
Better broadband needed to meet telehealth needs, USDA report warns
People who use telehealth services tend to live in urban areas and have more education and higher incomes, according to the agency's report.
By Meg Bryant • Nov. 8, 2018 -
Deep Dive
Red states say 'yes' to Medicaid; DME gets boost in Nevada
Three GOP-heavy states expanded Medicaid on Tuesday, and Democratic governor pickups in other parts of the country could further the trend. Elsewhere, durable medical equipment makers scored a win in Nevada.
By Rebecca Pifer Parduhn • Nov. 7, 2018 -
Abbott, J&J take part in FDA's NEST real world evidence pilot
The test cases will assess the role of RWE in proactive post-market surveillance, the evaluation of ablation devices and other activities across the medical device lifecycle.
By Nick Paul Taylor • Nov. 6, 2018 -
Pact to rush medical products for military clinched
The memorandum signed by the FDA and Department of Defense formalizes a program to speed review of high-priority treatments for the military such as freeze-dried plasma.
By Susan Kelly • Nov. 5, 2018 -
ACLA praises CMS for boosting data collection
The clinical lab trade group lauded the agency for taking steps to increase the number of laboratories submitting data informing Medicare payment rates.
By David Lim • Nov. 5, 2018 -
Deep Dive
Midterms 2018: Key health ballot votes in the states
It's not just Congress up for grabs in Tuesday's midterm elections. From Medicaid expansion to dialysis treatments to nurse staffing ratios, here's a snapshot of key votes, the odds and what they could mean for the industry.
By David Lim , Tony Abraham , Rebecca Pifer Parduhn , Samantha Liss • Nov. 2, 2018 -
CMS finalizes DME bidding program overhaul despite cost
Administrator Seema Verma said the final rule will increase patient access to medical products, but an August GAO report found beneficiaries are able to get needed items under the current paradigm.
By David Lim • Nov. 2, 2018 -
Patient deaths following Getinge device shutdowns prompt FDA warning
The agency has reports of three patient deaths following intra-aortic balloon pump malfunctions.
By Nick Paul Taylor • Nov. 2, 2018 -
FDA warns about Roche test after reports of patient strokes
Two patients suffered serious injuries involving strokes, prompting a warning that inaccurate results from the Swiss pharma's CoaguChek could cause serious injuries or death when taken with the blood thinner warfarin.
By Nick Paul Taylor • Nov. 2, 2018 -
Zimmer gets FDA nod for knee implant as portfolio strategy advances
Clearance coincided with first use of the device maker's knee surgery robot. The CEO has been vague about how it will differentiate its knee and spine devices from rival products, such as those sold by Mazor Robotics and Stryker.
By Nick Paul Taylor • Nov. 1, 2018 -
23andMe wins FDA clearance for first-of-its-kind genetic test
The agency also sent a safety communication warning about the danger of relying on genetic tests that have not been reviewed by FDA making claims about patient response to individual medications.
By David Lim , Nick Paul Taylor • Nov. 1, 2018 -
A timeline of Anthem's bold payment policies (and the fallout)
The payer's aggressive moves to push procedures like imaging to outpatient settings and taking a hard line on emergency room visits has drawn fire from doctors and other critics.
By Samantha Liss • Oct. 31, 2018 -
HHS opens cybersecurity coordination center after troubled year
It comes five months after a bipartisan group of lawmakers took HHS to task over stuttering attempts to coordinate cybersecurity information sharing.
By Nick Paul Taylor • Oct. 31, 2018 -
AdvaMed pitches new Anti-Kickback Statute safe harbors
The trade group wants the carve-outs for value-based pricing, warranty and risk-sharing arrangements.
By David Lim • Oct. 30, 2018 -
Deep Dive
5 sticking points in the EU medical device reg transition
Brexit and notified body capacity are among the uncertainties, as many in the medtech sector are uninformed.
By Meg Bryant • Oct. 29, 2018 -
Speedy Medicare breakthrough device coverage plan slated for March
AdvaMed has been pushing the agency to take steps to limit the delay between FDA approval and reimbursement.
By Nick Paul Taylor • Oct. 26, 2018 -
Novarad augmented reality system for surgery clears FDA
The OpenSight system is designed for use with Microsoft HoloLens technology.
By Meg Bryant • Oct. 25, 2018 -
Medtronic's dose-controlling app for pain pump gets FDA nod
The technology enables users to deliver doses on demand to cope with breakthrough pain.
By Nick Paul Taylor • Oct. 25, 2018 -
FDA clears Ansh Labs menopausal status test
Physicians can use the results to inform actions to prevent osteoporosis and heart disease.
By Nick Paul Taylor • Oct. 25, 2018 -
Adverse events prompt FDA to advise monitoring of Alcon implants
For now, the FDA says the problem looks to be limited to Alcon's implant. Glaukos markets a rival iStent device and saw its stock price increase by 40% when Alcon withdrew its stent.
By Nick Paul Taylor • Oct. 25, 2018 -
LabCorp blames data breach, hurricane for substandard Q3
The diagnostics branch was "particularly disappointing," said chairman and CEO Dave King.
By Rebecca Pifer Parduhn • Oct. 24, 2018