Recalls
-
 Retrieved from Fresenius Kabi on March 03, 2026
Retrieved from Fresenius Kabi on March 03, 2026
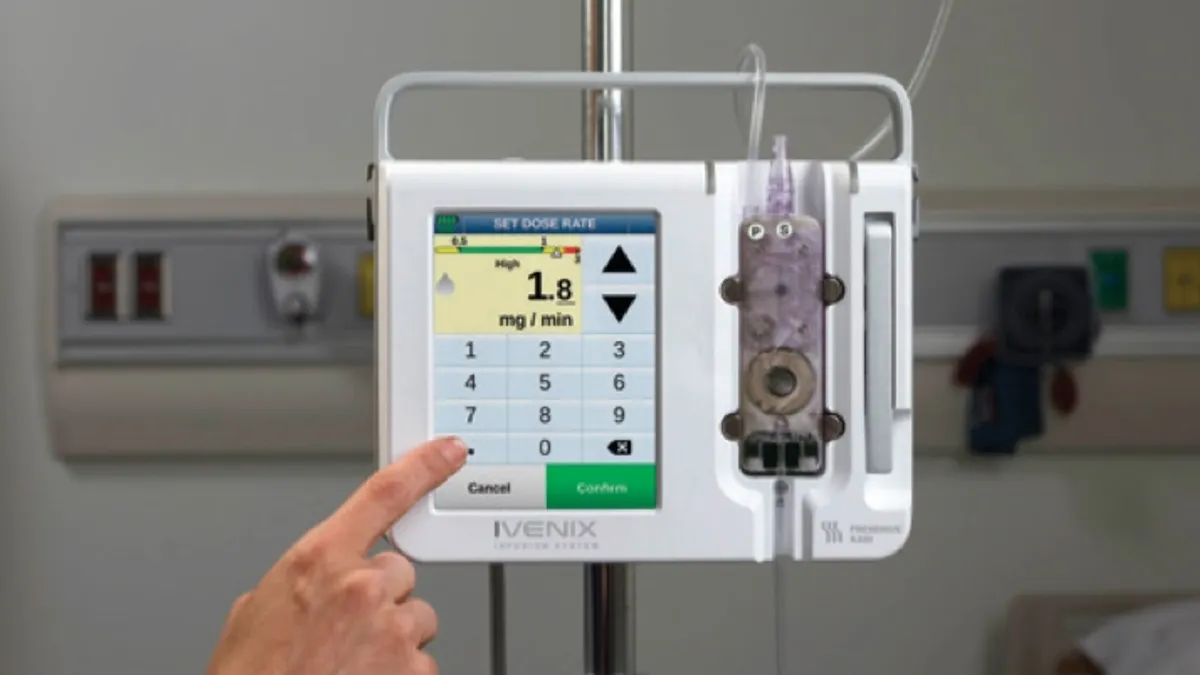
Fresenius Kabi recalls Ivenix infusion pumps over software problem
Fresenius has issued a software release to address issues that could interrupt treatment with its Ivenix large volume pumps.
By Elise Reuter • March 3, 2026 -
FDA posts early alert for safety issue with Impella heart pump devices
The cassettes have an increased risk of purge leaks that can lead to the loss of hemodynamic support.
By Nick Paul Taylor • Feb. 26, 2026 -
Medline addresses bed fire risk linked to death
The FDA said hand pendants and cords may overheat, causing the devices to spark, burn, melt, smoke and catch fire.
By Nick Paul Taylor • Feb. 18, 2026 -
J&J recalls coil systems used in aneurysm treatment
Healthcare providers are advised to stop using the devices after a failure to detach was linked with four serious injuries and one death.
By Susan Kelly • Feb. 6, 2026 -
Integra recalls wound and burn devices amid reports of serious injuries
Packaging failures could lead to breaches in the sterile barrier and patient infections.
By Nick Paul Taylor • Jan. 23, 2026 -
Boston Scientific recalls stent over issue linked to 3 deaths
The company said the deaths were associated with off-label or investigational use of the device.
By Nick Paul Taylor • Jan. 20, 2026 -
FDA posts Class I recall notice about Medtronic heart vent catheters
The company received three complaints about patients who had perforation injuries linked to the devices.
By Nick Paul Taylor • Dec. 23, 2025 -
FDA needs more staff, authority to oversee device recalls, watchdog finds
“FDA's challenges — such as insufficient staffing — can create inefficiencies in the process and potentially put lives at risk,” the U.S. Government Accountability Office said.
By Elise Reuter • Dec. 15, 2025 -
Olympus reinforces advice over device issue linked to 113 serious injuries
The company received complaints that the ligation loop can become unintentionally anchored in place.
By Nick Paul Taylor • Dec. 4, 2025 -
FDA flags risk from dropped BD Alaris pumps after 2 injury reports
BD told customers to immediately remove from use any devices they drop or severely jar.
By Nick Paul Taylor • Dec. 2, 2025 -
Abbott issues correction for millions of glucose sensors
The problem applies to Abbott’s Libre 3 and Libre 3 Plus sensors. The company said it has received reports of 736 severe adverse events and seven deaths.
By Elise Reuter • Nov. 24, 2025 -
Dexcom recalls G6 CGM app over software problem
A software defect could cause the app to terminate, resulting in missed alerts or alarms. Dexcom required users to download an update.
By Elise Reuter • Nov. 4, 2025 -
Cybersecurity triggers another recall of J&J’s Impella heart pump controller
While no patients have been harmed, the FDA categorized the action as a Class I recall because of the potential for serious injury or death.
By Nick Paul Taylor • Oct. 14, 2025 -
FDA expands early alert program to cover all medical devices
After a successful pilot, the agency has lifted the limitations on when it will provide early information about potentially high-risk safety events.
By Nick Paul Taylor • Sept. 30, 2025 -
J&J’s Abiomed starts third heart pump controller recall since June
As of Aug. 27, the company had reported five serious injuries and no deaths associated with the issue.
By Nick Paul Taylor • Sept. 24, 2025 -
Olympus removes needles after patient death
Olympus is recalling certain lots of needles, used for lung cancer biopsy, because components can detach during procedures.
By Elise Reuter • Updated Oct. 1, 2025 -
Exactech agrees to $8M settlement over defective knee implants
The payment resolves allegations brought by the Department of Justice that Exactech violated the False Claims Act by selling knee implants with components that failed prematurely.
By Elise Reuter • Sept. 19, 2025 -
FDA posts early alert about Abbott’s Tactiflex Ablation Catheter
Catheter tips came off three damaged devices during surgery but none of the patients suffered serious injuries or died.
By Nick Paul Taylor • Updated Sept. 22, 2025 -
BD expands Class I recall to cover 15 more Alaris pump infusion sets
BD, which has not received any complaints related to the issue, previously discontinued the devices affected by the expanded recall.
By Nick Paul Taylor • Sept. 17, 2025 -
AI devices with no clinical validation tied to more recalls, study finds
Public companies, which accounted for about half of AI-enabled devices on the market, had a higher rate of recalls and a lower rate of clinical evidence, according to a JAMA study.
By Elise Reuter • Sept. 2, 2025 -
J&J removes some Abiomed heart pump controllers from market
Abiomed reported one death associated with a pump driver circuit assembly that does not meet current specifications. The recall affects 69 controllers, a J&J spokesperson said.
By Susan Kelly • Aug. 28, 2025 -
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
By Nick Paul Taylor • Aug. 25, 2025 -
Medtronic recall of heart vent catheters tied to 3 serious injuries
The FDA said the request to quarantine the devices followed reports of the products “resisting shape retention when being bent.”
By Nick Paul Taylor • Aug. 19, 2025 -
Draeger removes ventilation filters over misleading carbon dioxide readings
Hospitals are being advised to stop using the filters after serious injuries were reported related to the problem, the FDA said in a recall notice.
By Elise Reuter • Aug. 12, 2025 -
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
By Nick Paul Taylor • Aug. 8, 2025