Page 3
-
Top medtech conferences in 2026
The medical device industry has a jam-packed year of events for professionals to attend, including the J.P. Morgan Healthcare Conference, DeviceTalks Boston and AdvaMed’s annual medtech meeting.
-
Convatec plans $1B investment in R&D in the US and UK
Capacity at the company's Boston site will increase by 50% as part of a $600 million, 10-year commitment to the U.S.
-
Revvity, Sanofi team on early Type 1 detection; Kihealth raises funds for diabetes test rollout
The Sanofi partnership will support clinical validation and regulatory submissions for Revvity’s new assay. Elsewhere, Kihealth raised $5 million in seed funding to launch its early detection test.
-
Q&A
Hospital for Special Surgery’s Ashis Barad says AI must solve real problems
Barad, chief digital and technology officer at HSS, outlined his approach to evaluating AI tools and how the orthopedic hospital uses the technology.
-
Medtronic wins CE mark for Penditure LAA exclusion device
The company plans to launch the device in Europe late next week, providing competition for AtriCure’s AtriClip product.
-
Deep Dive
AI could transform healthcare. Can safety-net providers keep up?
Implementing artificial intelligence requires significant human labor and technical expertise, threatening to create a digital divide between highly resourced health systems and safety-net providers.
-
CDRH shares regulatory guidance priorities for the coming 12 months
Use of real-world evidence to support regulatory decisions and predetermined change control plans for medical devices are two areas of focus for the agency.
-
SS Innovations changes course on FDA filing for soft tissue robot
A 510(k) submission now planned for this quarter sets up a potentially faster clearance than the de novo pathway originally planned, the company said.
-
Boston Scientific aims for market leadership in electrophysiology
The company has now treated 500,000 patients with its pulsed field ablation system, in a competitive category poised for growth.
-
Distalmotion adds hysterectomy indication; AiM raises funds for neurosurgical robot
The clearance gives Distalmotion, which sees opportunity in ambulatory settings, a third robotic procedure category in the U.S. Elsewhere, AiM Medical Robotics raised $8.1 million.
-
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
-
Boston Scientific unveils plans for new Watchman device
The company hopes to launch its next-generation Watchman system in the second half of 2027 or early 2028.
-
Medtech firms splitting into ‘haves’ and ‘have-nots’: EY
The number of medtech funding rounds has declined in recent months, but the value of overall deals has increased, according to a new report from EY.
-
HHS plans to furlough more than 40% of staff if government shuts down
Under the contingency plan, the CMS would lose 47% of its staff, while the CDC and the NIH would lose 64% and 75%, respectively.
-
FDA expands early alert program to cover all medical devices
After a successful pilot, the agency has lifted the limitations on when it will provide early information about potentially high-risk safety events.
-
EssilorLuxottica wins FDA OK for myopia-slowing eyeglass lenses
Investigators saw a 71% reduction in myopia progression in children who used the lenses for two years.
-
MedTech Europe pressures EU to make regulatory changes by early 2026
The request includes a call to postpone re-certification requirements for devices already certified under the medical technology regulations.
-
SS Innovations hires new CFO
Naveen Kumar Amar joins the surgical robot developer as it prepares to expand globally, including in Europe and the U.S.
-
Biolinq gets FDA de novo nod for intradermal glucose sensor
The device, called Biolinq Shine, is indicated for people with Type 2 diabetes who don’t depend on insulin.
Updated Sept. 29, 2025 -
Section 232 probe reignites tariff uncertainty for medtech firms
The Trump administration has used the investigations in other sectors to implement tariffs, but the earliest any potential actions would take place is summer 2026, analysts said.
-
Q&A
Que Dallara on Medtronic’s diabetes spinoff, pipeline and Abbott partnership
Dallara, president of Medtronic’s diabetes business, discussed the segment’s turnaround and why she sees a benefit in becoming a standalone firm.
-
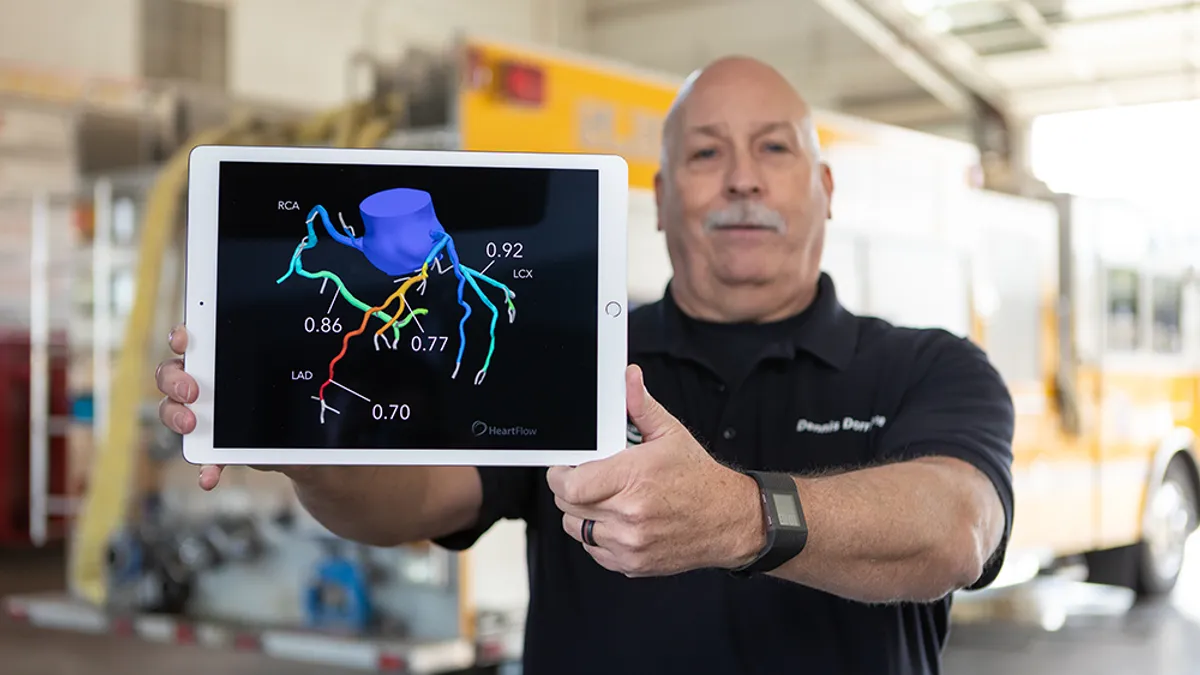
Heartflow receives FDA clearance for updated plaque analysis platform
The updated algorithm shows a 21% improvement in plaque detection, compared to the original version.