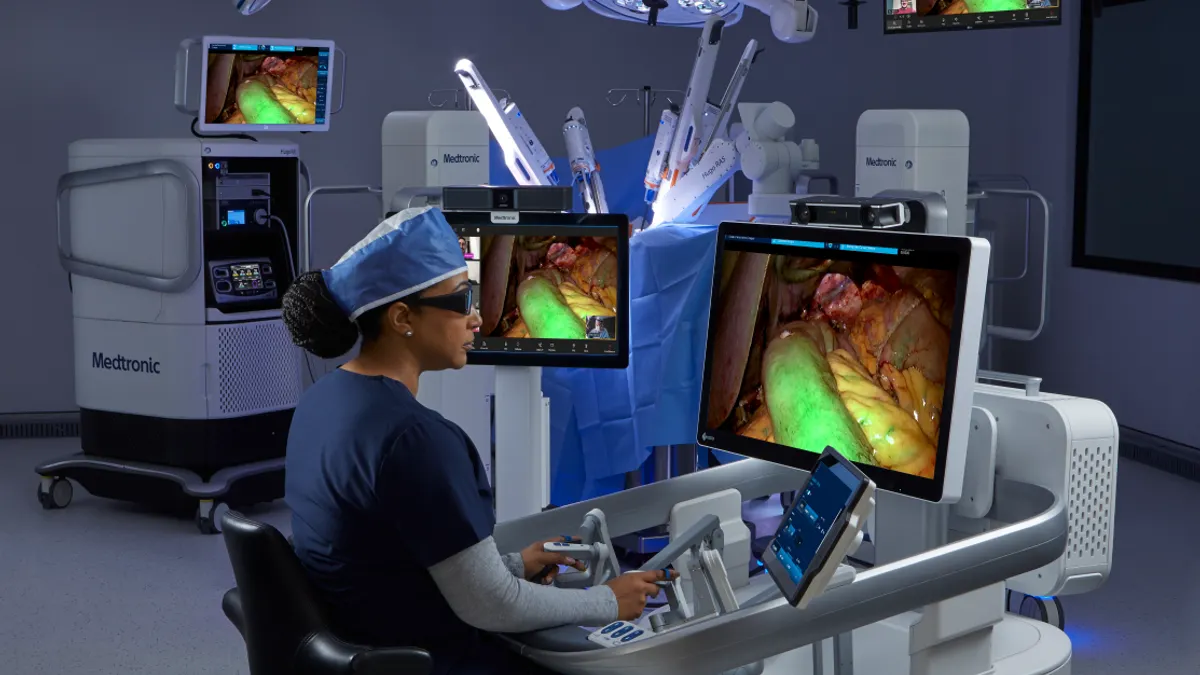
Medtronic said Tuesday an expansion of its London office will create the company’s largest global digital center for AI and robotics in surgery.
The effort builds on local partnerships in the research and design of robotic-assisted surgery capabilities. The expansion doubles both the size of the office, to 25,000 square feet, and the London workforce, to more than 200 people.
The Medtronic Centre of Digital Excellence develops specialized computing and software for hospital operating rooms and has launched hundreds of AI algorithms to help surgeons access analytics after performing surgery, the company said. A mock robotic operating theater allows testing of new technologies on site.
The digital technology supports surgical teams in planning, learning and collaboration with other experts, including through secure livestreaming. Capabilities in development include AI-powered decision support for surgeons while they operate in real time.
Medtronic sells its Hugo soft tissue robotic system in more than 30 countries, including in the U.K., and expects the robot to enter the U.S. market later in its current fiscal year, which ends in April 2026.
FDA grants breakthrough designation to Virtuoso robot
Virtuoso Surgical said Monday its robotic system has received the Food and Drug Administration’s breakthrough device designation for bladder lesion removal with en bloc resection.
The technique removes the specimen intact, increasing the accuracy of cancer staging and resulting in more definitive diagnoses than standard bladder tumor resection, the company said.
En bloc resection performed manually has been shown to lower cancer recurrence, according to Virtuoso, but the method is complex for surgeons to learn and perform. The company said its robotic system aims to enable wider adoption of en bloc resection by facilitating the approach.
Using needle-sized robotic arms, the Virtuoso system is designed to improve surgeons’ dexterity and precision in performing difficult procedures. The company said its manipulators are smaller than other robotic surgical instruments, allowing the surgeon to fit tools into previously unreachable areas of the body.
The FDA’s breakthrough device program prioritizes review of manufacturer submissions and is intended to speed development, assessment and review for authorization.
In May, Virtuoso said its system was used to perform bladder lesion excision in six patients during the pilot phase of the Viable trial at The Chinese University of Hong Kong.