Cardiac
-
Edwards focuses on earlier TAVR adoption
The heart valve specialist reported a second straight quarter of double-digit TAVR sales growth and credited recent study results with driving a “sense of urgency” for treating patients.
By Susan Kelly • Feb. 11, 2026 -
4 takeaways from the 2026 AF Symposium
Johnson & Johnson, Boston Scientific and Abbott were among the companies showcasing new data on atrial fibrillation treatments, including pulsed field ablation catheters.
By Susan Kelly • Feb. 10, 2026 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Abbott details Volt, TactiFlex data; Pulse Biosciences’ results shine
Abbott and Pulse released their findings at the AF Symposium that wrapped this weekend in Boston.
By Susan Kelly • Feb. 9, 2026 -
FDA breakthrough program starts FY2026 at steady pace
Orthopedics was the most active area over the second half of 2025, with the FDA issuing 13 breakthrough designations to devices in the therapeutic area.
By Nick Paul Taylor • Feb. 9, 2026 -
J&J corrects Impella heart pumps over issue linked to 22 serious injuries
The urgent correction notice is the latest in a series of actions related to the safety of Impella devices.
By Nick Paul Taylor • Feb. 4, 2026 -
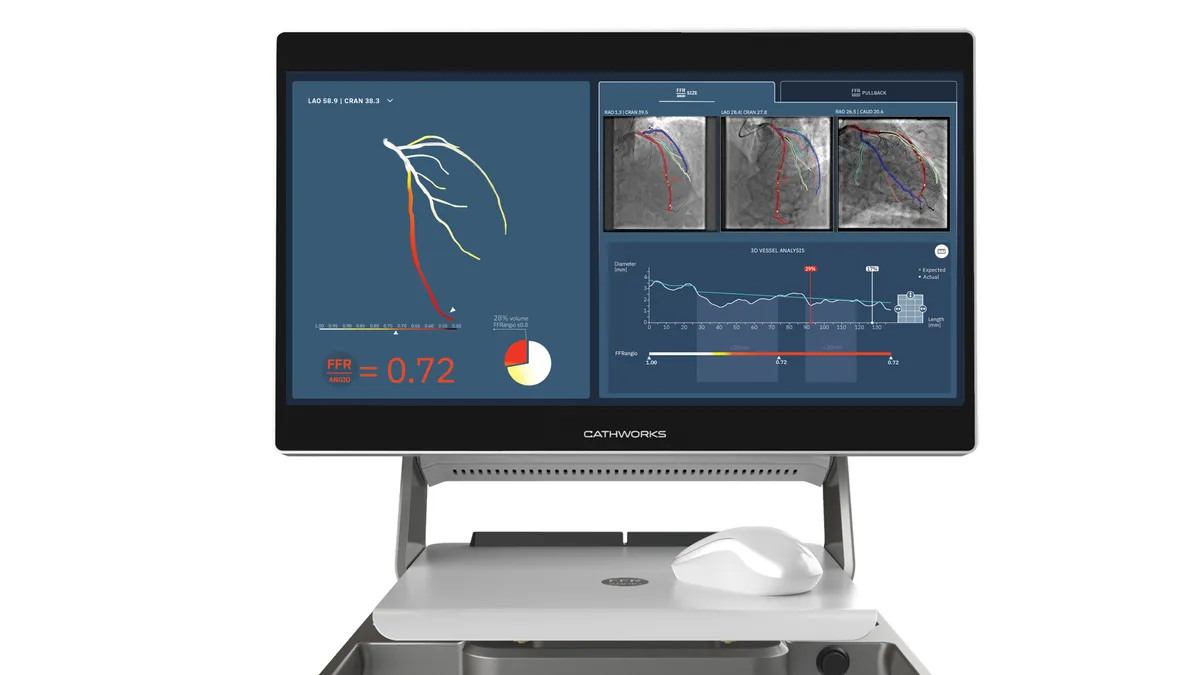
Medtronic to acquire CathWorks for up to $585M
The purchase will give Medtronic a larger presence in the cath lab, with a tool to help diagnose and treat coronary artery disease.
By Elise Reuter • Feb. 3, 2026 -
Edwards’ request for broader TAVR Medicare coverage gets pushback
Several medical societies supported extending Medicare coverage for TAVR to patients with asymptomatic aortic stenosis, but some physicians and rival Medtronic said more data is needed.
By Susan Kelly • Jan. 28, 2026 -
Deep Dive
5 robotic surgery trends to watch in 2026
Soft tissue robotic systems designed to improve surgical precision are expanding into new specialties and increasing competition for longtime industry leader Intuitive Surgical.
By Susan Kelly • Jan. 27, 2026 -
Medtronic wins CE mark for single-shot pulsed field ablation catheter
Medtronic’s Affera Sphere-360 is a single-shot device, putting it in direct competition with Boston Scientific’s Farawave catheter.
By Nick Paul Taylor • Jan. 26, 2026 -
Intuitive’s cardiac initiative includes valve repair, LAA closure
With nine new procedures cleared for the da Vinci 5 robot, Intuitive’s strategy includes working with a limited number of U.S. sites this year to establish cardiac programs.
By Susan Kelly • Jan. 26, 2026 -
Abbott posts Q4 sales below expectations
Pricing challenges in Abbott’s nutrition business and lower than expected sales of continuous glucose monitors drove down the company’s shares.
By Elise Reuter • Jan. 22, 2026 -

 Retrieved from Anteris Technologies Global on January 21, 2026
Retrieved from Anteris Technologies Global on January 21, 2026
Medtronic to buy up to $90M stake in heart valve developer Anteris
Medtronic’s investment is contingent on Anteris completing a proposed $200 million public stock offering.
By Susan Kelly • Jan. 21, 2026 -
Abbott gets Europe’s CE mark for dual ablation catheter
The device can deliver both radiofrequency energy and pulsed field ablation to treat heart arrhythmias.
By Elise Reuter • Jan. 20, 2026 -
Boston Scientific to acquire Penumbra for $14.5B
With the planned purchase, Boston Scientific will gain several thrombectomy devices to remove clots from blood vessels.
By Elise Reuter • Jan. 15, 2026 -
Boston Scientific gains FDA’s OK for new PFA catheter
The Farapoint device adds another tool to the company’s pulsed field ablation portfolio that could help increase or sustain its electrophysiology market share, one analyst said.
By Susan Kelly • Jan. 14, 2026 -
Edwards calls off JenaValve buyout after court halts deal
Edwards terminated the deal after the Federal Trade Commission moved to block the acquisition due to anticompetitive concerns.
By Susan Kelly • Jan. 12, 2026 -
Teleflex CEO leaving company
Stuart Randle, who retired in 2018, has replaced Liam Kelly as CEO and president on an interim basis.
By Nick Paul Taylor • Jan. 9, 2026 -
Stereotaxis wins FDA approval for robotically navigated ablation catheter
Stereotaxis’ CEO said the company has been “hampered clinically, commercially and strategically” by its prior dependence on a J&J catheter.
By Nick Paul Taylor • Jan. 7, 2026 -
Biobeat raises $50M to commercialize cuffless blood pressure monitor
The company will use the money to try to establish its cuffless device as a go-to option for 24-hour blood pressure monitoring in the U.S.
By Nick Paul Taylor • Jan. 6, 2026 -
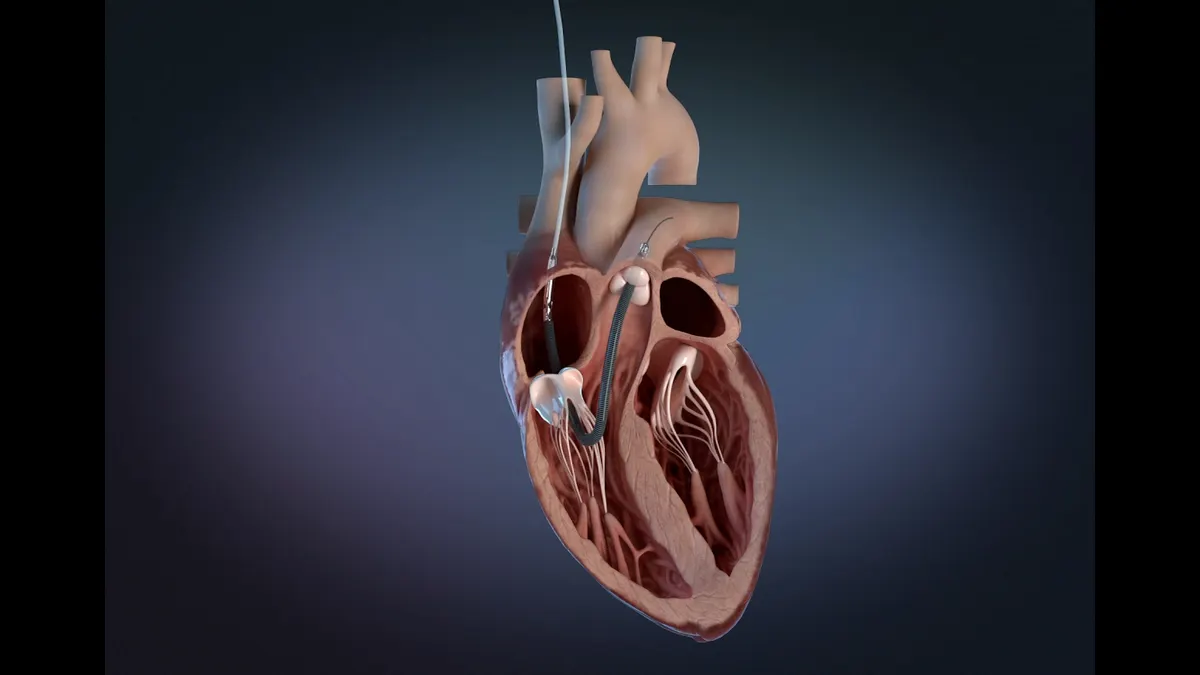
Edwards receives FDA approval for mitral valve replacement system
The Sapien M3 device is the first approved mitral regurgitation treatment to use a transseptal approach, Edwards said.
By Elise Reuter • Dec. 23, 2025 -
FDA posts Class I recall notice about Medtronic heart vent catheters
The company received three complaints about patients who had perforation injuries linked to the devices.
By Nick Paul Taylor • Dec. 23, 2025 -
Abbott receives FDA approval for Volt PFA system
Abbott can now compete with Medtronic, Boston Scientific and J&J in the U.S. for the fast-growing pulsed field ablation market.
By Elise Reuter • Dec. 22, 2025 -
Abbott receives clearance for heart delivery device in premature babies
Physician feedback has informed a delivery device designed to make procedures safer and easier.
By Nick Paul Taylor • Dec. 22, 2025 -
Pulse Biosciences wins FDA approval to begin PFA catheter study
Shorter, nanosecond pulses for treating atrial fibrillation differentiate the company’s approach from existing pulsed field ablation systems on the market.
By Susan Kelly • Dec. 18, 2025 -
CMS considers expanding TAVR coverage to asymptomatic patients
Medicare reimbursement for aortic stenosis patients without symptoms could accelerate procedure growth for Edwards Lifesciences, which requested the CMS review, analysts said.
By Susan Kelly • Dec. 16, 2025