COVID-19: Page 2
-
Interest in elective surgeries up 115% over pre-COVID levels, Google searches show: Needham
The rise in searches correlates with an increase in the size of the market for elective procedures, Needham analysts report, signaling better times for medtech companies.
By Elise Reuter • June 15, 2023 -
Cue Health wins first non-emergency authorization for COVID test, days after Goldman downgrade
The FDA’s marketing authorization for Cue’s COVID-19 molecular test could boost consumer access, but the company faces strong competition from more established diagnostic firms.
By Peter Green • June 7, 2023 -
Cue Health’s inability to find post-COVID markets for testing prompts Goldman downgrade
The analysts warned switching to non-COVID-19 tests will mean entering a competitive market “where larger companies with financial resources have an established installed base.”
By Nick Paul Taylor • June 5, 2023 -
CDC Director Walensky to step down in June
Rachel Walensky, who has served as director since 2001, was criticized for the federal government’s handing of the pandemic, announcing in August last year that she planned to reorganize the CDC.
By Sydney Halleman • May 5, 2023 -
Opinion
Ending free COVID tests risks worsening the pandemic
If Americans start paying out of pocket for COVID tests, they'll test much less — if at all, argue representatives of the Testing at Home Coalition.
By Amy Kelbick and Eric Zimmerman • April 20, 2023 -
Intuitive posted 26% jump in robotic procedures in Q1 as hospitals chipped away at backlog
The company experienced “some backlog effect from patients generally returning to more normalized healthcare routines, given the effect of the pandemic over several years.”
By Nick Paul Taylor • April 19, 2023 -
Medtech sector poised for growth as healthcare staffing tops pre-COVID levels: KeyBanc
The analysts are “leaning into increased medtech exposure” after determining that several challenges that have constrained the industry are easing.
By Nick Paul Taylor • April 13, 2023 -
FDA sets end date for raft of COVID-related shortages that began early in pandemic
Shortages of home-use ventilators and clinical sample concentrators are expected to continue.
By Nick Paul Taylor • March 20, 2023 -
QuidelOrtho COVID test is first to win FDA nod via premarket review
Special controls instituted by the FDA as part of the De Novo assessment pave the way for other tests to come to market without pandemic-era Emergency Use Authorization.
By Nick Paul Taylor • March 9, 2023 -
FDA authorizes first at-home flu-COVID-19 combination test days after its developer files for bankruptcy
Lucira filed for bankruptcy protection last week, saying the “protracted” authorization process caused it to miss out on test sales in the 2022 to 2023 flu season.
By Nick Paul Taylor • Feb. 27, 2023 -
Henry Schein Q4 profit drops, hurt by falling sales of COVID test kits, PPE
Flu and COVID-19 cases increased appointment cancellations and added to staffing shortages in the quarter.
By Nick Paul Taylor • Feb. 17, 2023 -
Baxter, Abbott, Thermo Fisher among medtech firms cutting jobs on supply issues, slide in testing
"What COVID gave to diagnostics companies, COVID taketh away now that the virus is endemic,” said one analyst.
By Susan Kelly • Updated Feb. 13, 2023 -
Thermo Fisher cuts additional 230 jobs in California
The job reductions come amid a downturn in demand for COVID-19 tests.
By Susan Kelly • Updated Feb. 10, 2023 -
Medtech earnings highlights: Easing of macro pressures, accelerated growth in 2023
Still, the latest round of quarterly results show that hospital staffing and supply chain remain a challenge.
By Nick Paul Taylor • Feb. 6, 2023 -
Quest cuts 2023 COVID tests forecast amid continued slump in demand; Q4 profit plunges
The company performed 1.2 million molecular tests in the fourth quarter, down from 1.9 million in the third quarter and 5.4 million one year ago.
By Nick Paul Taylor • Feb. 3, 2023 -
FDA weighs shift in COVID vaccination strategy
Agency scientists are proposing to update COVID shots once a year to match circulating coronavirus strains, as well as simplifying current vaccination regimens.
By Ned Pagliarulo • Jan. 23, 2023 -
Investors hand Vaxxas $23M to support trial of needle-free COVID-19 vaccine
With support from the Gates Foundation, the needle-free technology could get more people vaccinated and avoid cold-chain issues.
By Nick Paul Taylor • Dec. 12, 2022 -
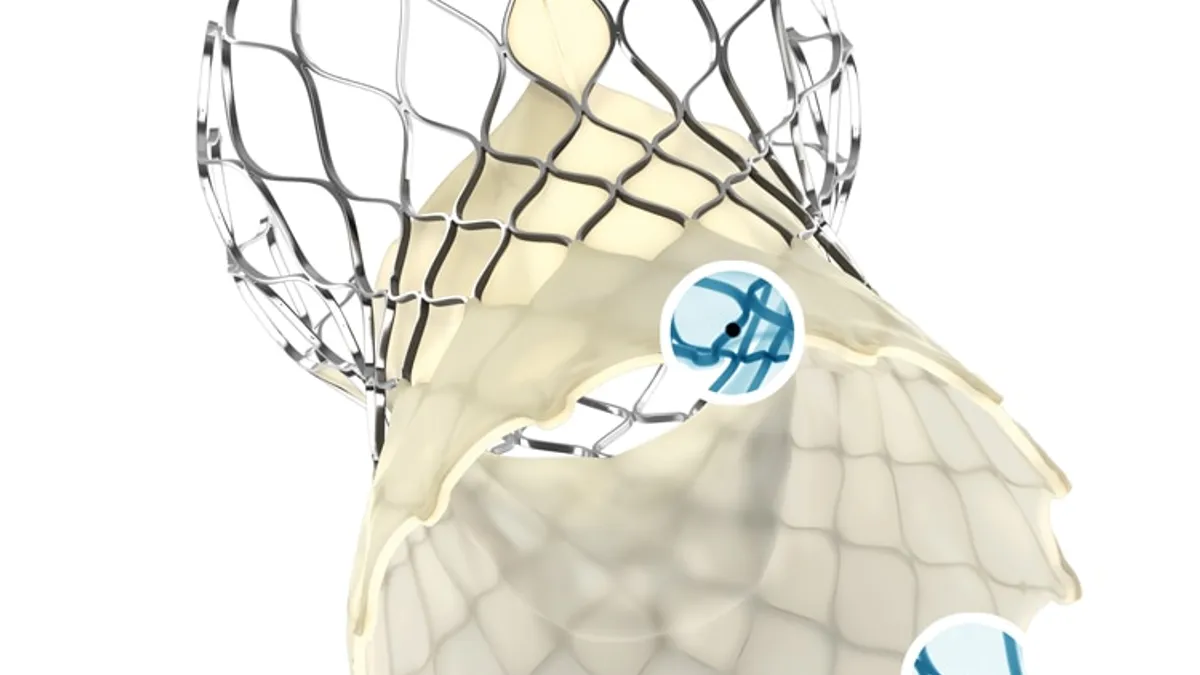
 Retrieved from Medtronic on September 19, 2022
Retrieved from Medtronic on September 19, 2022
Excess deaths to continue constraining recovery of TAVR market in 2023: Goldman
Hospital staffing shortages and excess deaths among seniors are expected to slow the recovery of structural heart procedures, Goldman Sachs analysts said.
By Nick Paul Taylor • Dec. 7, 2022 -
18M projected to lose Medicaid coverage at end of COVID-19 emergency
Many people who are currently enrolled in Medicaid will transition to other coverage, but 3.8 million people will completely lose insurance, according to the Robert Wood Johnson Foundation.
By Susan Kelly • Dec. 6, 2022 -
The 10 biggest medtech stories of 2022
MedTech Dive reporting this year has explored how companies maintained momentum even amid supply shortages and rising inflation rates.
By MedTech Dive staff • Dec. 3, 2022 -
Masimo’s pulse oximeter has no significant racial bias, study finds
The company said the result reflects its use of four additional signal processing engines that cut the impact of factors including skin pigmentation.
By Nick Paul Taylor • Nov. 28, 2022 -
‘Tripledemic’ concerns spur FDA to issue emergency authorization for flu-COVID-19 combination test
Experts have warned “a viral hurricane is making landfall” as cases of RSV, influenza and COVID-19 rise simultaneously.
By Nick Paul Taylor • Nov. 23, 2022 -
Q3 Earnings Wrap: Bumpy time for medtech with inflation, FX, supply woes
Macroeconomic pressures were a persistent theme for the industry, with workforce reductions a part of the fallout for some companies.
By Susan Kelly • Nov. 21, 2022 -
How Labcorp, Abbott, BD, Siemens plan to expand home testing market
As demand wanes for COVID-19 testing, businesses see an opportunity in direct-to-consumer diagnostics.
By Elise Reuter • Nov. 16, 2022 -
Roche starts OTC sales of COVID-19 test in first push into US market
CVS, Amazon and the Optum Store are stocking the product, rebranded Pilot.
By Nick Paul Taylor • Nov. 11, 2022