COVID-19: Page 33
-
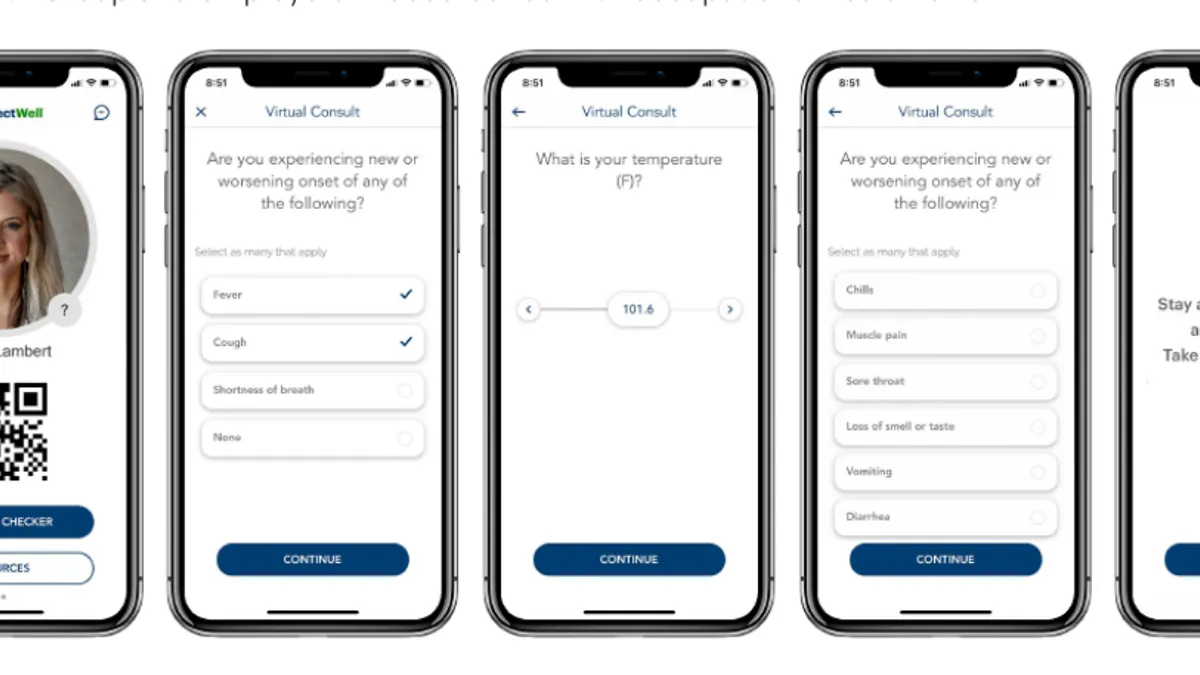
Health systems, ASCs see restart of elective care taking months despite steps to ease patient fears
The biggest worry among providers is the possibility of an outbreak or second wave, followed by low patient demand and inadequate supplies, according to a new survey from consulting firm Deloitte.
By Shannon Muchmore • May 28, 2020 -
EU group sets path for pre-MDR notified body renewal, coronavirus-era surveillance
A new guidance applies to designating authorities and medical device sector notified bodies whose designations would have expired between this week and the Medical Device Regulation's delayed go-live date next May.
By Maria Rachal • May 28, 2020 -
Emergency authorization granted to COVID-19 ICU prediction software
The FDA nod for CLEW Medical's system is among a series of regulatory OKs for products designed to inform care for infected patients.
By Nick Paul Taylor • May 28, 2020 -
FDA offers reference panel to validate coronavirus tests amid false negatives
The aim is to aid commercial and lab developers in ensuring diagnostic testing quality with independent performance validation. Recent studies flagged double-digit rates of false negatives among such tests.
By Nick Paul Taylor • May 28, 2020 -
Analysts saw 30% drop in TAVR growth in April, but Q2 may be better than feared
The Jefferies tracker suggests volume expectations for players in the transcatheter aortic valve replacement market, such as Edwards Lifesciences and Medtronic, may prove a bit conservative.
By Nick Paul Taylor • May 27, 2020 -
CDC seeks to limit false positives with COVID-19 antibody testing, as immunity question lingers
The interim guidelines promote using high-specificity assays and conducting confirmatory tests when appropriate. However, the agency won't yet use serologic test results to sway public health recommendations.
By Nick Paul Taylor • May 27, 2020 -
Medtechs eye key role of ASCs in return of elective care
Elective procedures were increasingly moving from hospitals to lower-cost ambulatory surgery centers prior to COVID-19. Now, ASCs may have a new advantage as a setting where patients feel safer returning to care.
By Maria Rachal • May 26, 2020 -
ResMed, Medtronic embrace remote tech amid COVID-19, say it's here to stay
The crisis has sped adoption of digital health technologies to help hospitals create safe physical distances between healthcare workers and patients. Execs say some could become permanent products.
By Greg Slabodkin • May 26, 2020 -
Martha plots M&A, Hugo robot sees delay: Takeaways from Medtronic's Q4
Despite the negative impacts of COVID-19 on revenue in the latest quarter, the medtech giant has its strongest balance sheet in years and is looking for acquisition opportunities in the pandemic landscape.
By Maria Rachal , Greg Slabodkin • May 22, 2020 -
FDA eases rules on changes to PMA, HDE devices to avoid coronavirus supply disruptions
Companies that market medical devices under premarket approvals or humanitarian device exemptions can make limited modifications to design and production without giving prior notice, according to special guidance.
By Nick Paul Taylor • May 22, 2020 -
Abbott, Roche ink deals with UK for 10M coronavirus antibody tests
The notice from the government, which bought $20 million in unreliable antibody tests early in the crisis, came as FDA removed 28 products from a list of the tests that can be used without emergency use authorizations.
By Nick Paul Taylor • May 22, 2020 -
April was dismal for US hospitals, with long road ahead, surveys find
Operating margins plummeted 174% last month compared to April 2019 and were down 118% from March of this year as surgeries, ER visits and outpatient appointments declined drastically during the COVID-19 pandemic.
By Shannon Muchmore • May 21, 2020 -
Medtronic's revenue dropped 26% in Q4 as coronavirus hit broadly
New CEO Geoff Martha told investors Thursday nearly all sides of the business were negatively impacted by the pandemic's effects.
By Greg Slabodkin • May 21, 2020 -
Hospitals push FEMA to form broad supply pact with medtech
The agency on Thursday is set to discuss a proposed five-year voluntary agreement with industry to better distribute medical products, which health systems hope will go beyond personal protective equipment.
By Nick Paul Taylor • Updated May 26, 2020 -
AdvaMed, health systems set ground rules for return of sales reps
Hospitals, nurses and the medtech industry issued guidance to pave the way for device company representatives to re-enter healthcare facilities.
By Susan Kelly • May 20, 2020 -
COVID-19 drives EC to change rules on notified body designations
The European Commission is allowing certain deviations from normal procedures governing notified bodies, enabling renewal of designations under the outgoing device regulations without performing on-site assessments.
By Nick Paul Taylor • May 20, 2020 -
Labs welcome CMS rate for coronavirus antibody testing
Analysts at William Blair said the roughly $42 rate for common serological tests is higher than expected, which may bode well for antigen testing reimbursement.
By Nick Paul Taylor • May 20, 2020 -
Coronavirus pushes Mayo Clinic's operating income into free fall
Operating income plunged 88% by the end of the first quarter due to the pandemic and the near closure of the health system's outpatient business. Still, Mayo has become a leader in COVID-19 testing.
By Samantha Liss • May 19, 2020 -
Investors bet diabetes tech immune to full brunt of COVID-19
Pure play diabetes device makers aren't clear yet on how economic uncertainty and the transition to telemedicine may affect new patient demand, but are outperforming the stock market by a long shot.
By Maria Rachal • May 19, 2020 -
Philips lung ultrasound, Beckman sepsis diagnostic receive BARDA coronavirus backing
The separate projects aim to incorporate machine learning algorithms to enhance technologies meant to support COVID-19 patients.
By Susan Kelly • May 19, 2020 -
FDA OKs 1st at-home collection kit for use with multiple labs, coronavirus tests
The emergency use authorization to Everlywell follows earlier nods to LabCorp and Rutgers Clinical Genomics Laboratory for at-home specimen collection for analysis solely at their own labs.
By Nick Paul Taylor • May 18, 2020 -
UnitedHealth, Microsoft launch COVID-19 screening app for employers
The healthcare behemoth said it will control employees' medical data and manage opt-in and consent requirements for users. The app will not provide tracking or contact tracing information.
By Rebecca Pifer • May 15, 2020 -
FDA revokes umbrella EUA for infusion pumps due to lack of industry use
The agency told medtechs it may instead grant individual EUAs for the devices going forward. B. Braun contends it is the only company with such an emergency authorization and is not affected by the revocation.
By Greg Slabodkin • Updated Sept. 24, 2020 -
Abbott on defense as FDA flags false negative reports with point-of-care coronavirus test
An independent study circulated this week showed the rapid ID Now COVID-19 test could miss 48% of positive cases under some conditions. Abbott said late Thursday a backup test should be performed in certain cases.
By Nick Paul Taylor • May 15, 2020 -
Consumer COVID-19 fears, coverage concerns could stymie medtech rebound: Wall Street surveys
Some analysts say industry predictions on timing of a rebound in elective procedures may be too rosy.
By Maria Rachal • May 14, 2020