Diagnostics: Page 23
-
Core diagnostic growth offsets falling COVID-19 sales at Hologic, Thermo Fisher, Qiagen
Rising demand for core diagnostics offset better-than-feared declines in COVID-19 test sales, shedding light on the companies’ post-pandemic futures.
By Nick Paul Taylor • July 29, 2022 -
Labcorp lowers forecast after Q2 slowdown, will spin off drug development unit
A drop in COVID-19 testing contributed to the company’s second quarter decline, while its base business grew by 1.2% compared to last year.
By Ricky Zipp • July 28, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
MedTech Europe pushes for semiconductor prioritization as US moves to increase local supply
The trade group wants political action to stop patient harm from “a growing divergence” between semiconductor supply and demand.
By Nick Paul Taylor • July 27, 2022 -
Philips shares hit 10-year low as supply chain challenges weigh on revenue
Sleep apnea machine recall adds to woes as sales forecast is slashed for remainder of 2022.
By Elise Reuter • July 25, 2022 -
Johns Hopkins sepsis alert tool reduced patient deaths, studies find
Three prospective studies of a machine learning tool developed by a Johns Hopkins spinout found it led to earlier antibiotic treatment and reduced patient mortality.
By Elise Reuter • July 22, 2022 -
Q&A
Friday Q&A: Matthew Binnicker, Mayo Clinic's director of clinical virology, talks COVID-19, monkeypox testing
With cases of both viruses rising across the U.S., clinical laboratories are bringing in staff to ramp up monkeypox testing, even as demand falls for clinical diagnostics of COVID-19.
By Elise Reuter • July 22, 2022 -
Roche CEO Schwan, who guided company as cancer drug powerhouse, to step down next year
The drugmaker’s current diagnostics chief Thomas Schinecker will take over as CEO in March, while Schwan will seek election as chair of Roche’s board.
By Jonathan Gardner • July 21, 2022 -
Test makers urge House, Senate to include diagnostic reforms in user-fee bill
AdvaMedDx, Abbott and Roche are among companies asking lawmakers to address the “urgent public health issue.”
By Nick Paul Taylor • July 21, 2022 -
Quest Diagnostics raises 2022 revenue forecast amid continued demand for COVID testing
The company boosted its outlook for the diagnostics unit even as the division posted a 31% decline in revenue in the second quarter.
By Ricky Zipp • July 21, 2022 -
Abbott's Q2 sales boosted by COVID-19 testing; outlook for diagnostics demand remains uncertain
The company’s $2.3 billion in COVID-19 testing sales beat J.P. Morgan and Stifel analysts’ estimates by about $1 billion, while CEO Robert Ford cautioned that forecasting demand remains challenging.
By Ricky Zipp • July 20, 2022 -
Sonic Healthcare joins CDC's monkeypox testing push to further grow capacity
Providers can order the Sonic Healthcare test as they would any other service, opening another pathway to diagnose a virus that has now infected almost 2,000 people in the U.S.
By Nick Paul Taylor • July 19, 2022 -
Diagnostic sector faces a 'challenging set-up' amid economic slowdown: analysts
Goldman Sachs warned there will be “limited visibility over the course of the year,” adding that companies may need to focus more on demonstrating their paths to profitability.
By Nick Paul Taylor • July 19, 2022 -
Illumina fails to block EU antitrust review of $8B Grail merger
By siding with the European Commission, the General Court has cleared the EU to resume a review that could lead to it blocking the deal and fining Illumina.
By Nick Paul Taylor • July 18, 2022 -
Q&A
Friday Q&A: Ian Lipkin, Columbia epidemiologist, talks COVID-19 testing as U.S. sees another wave
Lipkin explained why he’s not a fan of antigen tests, and stressed the need to keep PCR testing a core part of the pandemic response.
By Elise Reuter • July 15, 2022 -
Abbott, Intuitive, J&J to kick off quarterly earnings season next week as recession threat looms
Earnings results are poised to set expectations for the impact of a potential slowdown and shed light on trends with implications for companies including Boston Scientific, Medtronic and Stryker.
By Nick Paul Taylor • July 15, 2022 -
Labcorp launches blood test for neurodegenerative disease biomarker
The test could detect Alzheimer’s before it manifests in routine imaging or clinical symptoms, helping physicians assess the effectiveness of treatments.
By Nick Paul Taylor • July 14, 2022 -
EC publishes IVDR guidance in absence of completed Eudamed database
The commission recently announced a timeline for complete implementation of Eudamed, aiming for the second quarter of 2024.
By Ricky Zipp • July 13, 2022 -
Deep Dive
With COVID testing on the wane, small diagnostics companies shift focus
Startups that grew quickly from large contracts are looking nervously to a new future, as demand plummets for mass testing and shifts to homes.
By Elise Reuter • July 13, 2022 -
Quest launches monkeypox PCR test as U.S. attempts to catch up with rising cases
The company estimates it will be able to process 30,000 tests per week by the end of July. As of July 12, there were 929 confirmed cases in the U.S., the CDC reported.
By Ricky Zipp • July 13, 2022 -
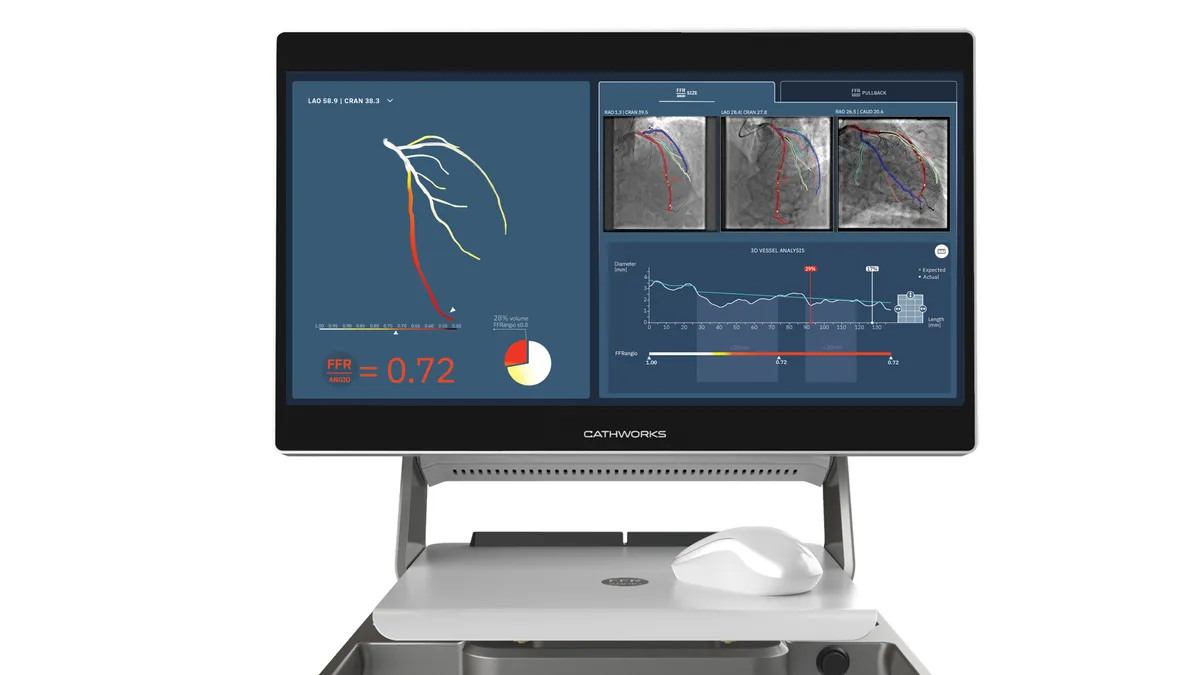
Medtronic lands option to buy artery disease player CathWorks for up to $585M
CathWorks has the right to compel Medtronic to complete the acquisition if it chooses not to exercise its option.
By Nick Paul Taylor • July 13, 2022 -
Hologic, Thermo Fisher roll out respiratory panels for Europe ahead of cold and flu season
Australia’s experience suggests Europe and other parts of the northern hemisphere may face high levels of respiratory disease this winter.
By Nick Paul Taylor • July 12, 2022 -
European Commission targets spring of 2024 for fully functional Eudamed database
The Commission is planning a two-phase transition to mandatory use of the database after it goes live.
By Nick Paul Taylor • July 11, 2022 -
Labcorp starts offering monkeypox testing using the CDC's PCR test
Labcorp claims it will become the first national laboratory to offer the test by beating its chief rival to market.
By Nick Paul Taylor • July 8, 2022 -
High-risk IVD tests rules finalized by European Commission despite industry calls for change
MedTech Europe pushed back against some of the requirements, but the Commission has retained disputed parts of the document.
By Nick Paul Taylor • July 8, 2022 -
SD Biosensor agrees to buy Meridian Bioscience for $1.53B in bid to enter U.S. IVD market
The Cincinnati-based diagnostics company says being acquired will help it enter new markets as part of SD Biosensor, as the COVID-detection business wanes.
By Elise Reuter • July 7, 2022