Diagnostics: Page 51
-
Hologic, LabCorp win emergency use nods as FDA eases rules to boost US coronavirus test volume
In some cases, manufacturers can distribute, and labs can use, new commercially developed tests prior to an emergency use authorization. FDA's updated policy also boosts states' authority to greenlight tests.
By Greg Slabodkin • March 17, 2020 -

 Official White House Photo by Andrea Hanks. (2020). "President Trump meets with the Coronavirus Task Force" [Photograph]. Retrieved from https://www.flickr.com/photos/whitehouse/49613832638/in/photostream/.
Official White House Photo by Andrea Hanks. (2020). "President Trump meets with the Coronavirus Task Force" [Photograph]. Retrieved from https://www.flickr.com/photos/whitehouse/49613832638/in/photostream/.
To fight coronavirus spread, high-throughput tests bring potential benefits, challenges
Deborah Birx, White House coronavirus response coordinator, said labs will just as importantly need supplies to ramp up testing. The American Clinical Laboratory Association is concerned about potential supply shortages.
By Greg Slabodkin • March 15, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Thermo Fisher coronavirus test gets FDA nod
Trump administration officials promised on Sunday that 2,000 labs would be running 1.9 million tests this week. Separately, the American Clinical Laboratory Association expects daily testing capacity to exceed 20,000 next week, with the potential to grow to 280,000 by April 1.
By Maria Rachal • March 15, 2020 -
Trump declares national emergency, opening up billions for coronavirus
The president also teased an imminent FDA emergency use authorization for a test from Thermo Fisher, with LabCorp and Quest saying they're collaborating with Roche.
By Samantha Liss • Updated March 13, 2020 -
Roche is 1st commercial coronavirus test maker to win FDA emergency use authorization
After FDA allowed the company to pre-position its test in the U.S., Roche said it's committed to "going to the limits" of its production capacity to churn out millions of tests per month.
By Maria Rachal • March 13, 2020 -
Natera breaks ties with Qiagen on NGS testing, citing Illumina deal
The companies teamed up two years ago to develop cell-free DNA assays for a Qiagen reader system, but Thermo Fisher-bound Qiagen later shifted course, which Natera said was a "material breach of the agreement."
By Nick Paul Taylor • March 13, 2020 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
CDC chief: Reagents critical to coronavirus tests 'now are in short supply'
Separately, the government Friday announced funding for development of COVID-19 diagnostic tests from DiaSorin Molecular and Qiagen meant to detect the pathogen in about an hour.
By Greg Slabodkin • March 13, 2020 -
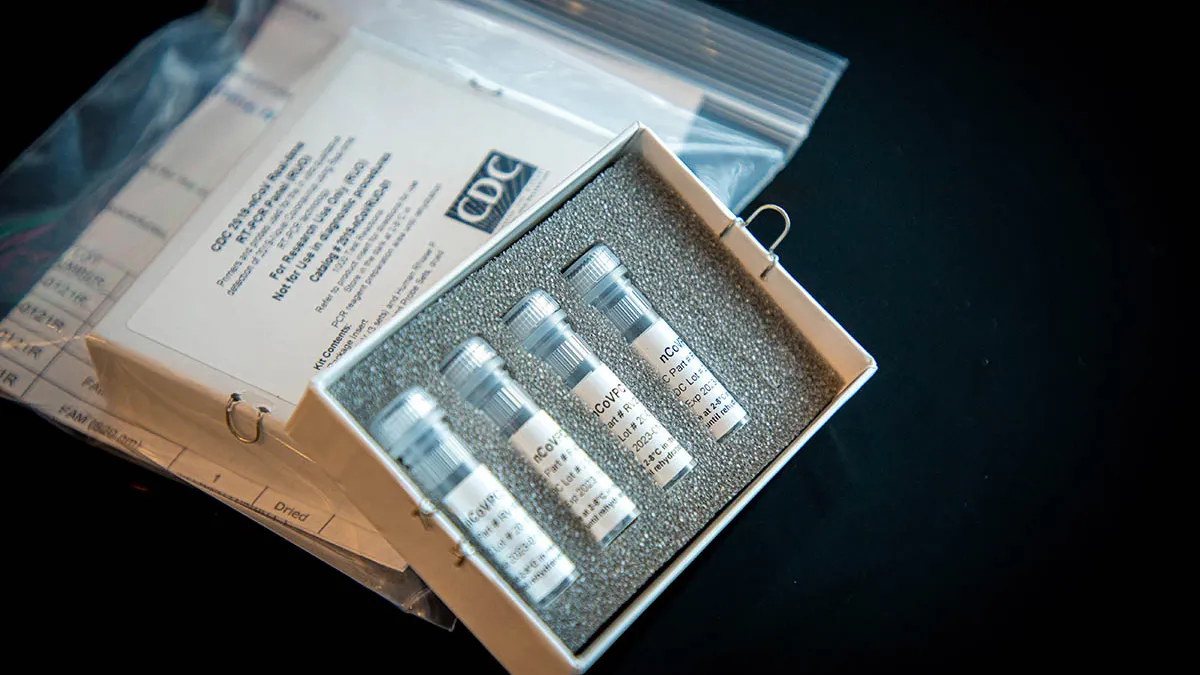
 U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
FDA chief warns of supply 'pressure' on reagents for coronavirus tests
Qiagen, a major supplier of RNA extraction kits, confirmed to MedTech Dive Thursday that "extraordinary demand for coronavirus testing workflows" is challenging the company's capacity.
By Greg Slabodkin • March 12, 2020 -

 U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
U.S. Centers for Disease Control. "CDC 2019-Novel Coronavirus (2019-nCoV) test kit". Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
White House, CDC under fire for availability of coronavirus tests
The Trump administration is facing criticism that federal agencies did not engage the big private labs early enough in the public health emergency.
By Greg Slabodkin • March 11, 2020 -
ECRI: Poor sterilization, failure to learn from device problems threaten patient safety
The 2020 list put together by the nonprofit ECRI Institute also highlights diagnostic errors as a top patient safety concern for a third consecutive year.
By Maria Rachal • March 10, 2020 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel coronavirus SARS-CoV-2" [Microscope image]. Retrieved from https://www.flickr.com/photos/nihgov/49535193876/in/album-72157713108522106/.
Labs step up capacity to meet demand for nationwide coronavirus testing
The Trump administration is relying on the "enormous capacity" of commercial labs to enable wide availability of COVID-19 diagnostic testing to the American public through physicians offices and pharmacies.
By Greg Slabodkin • March 10, 2020 -
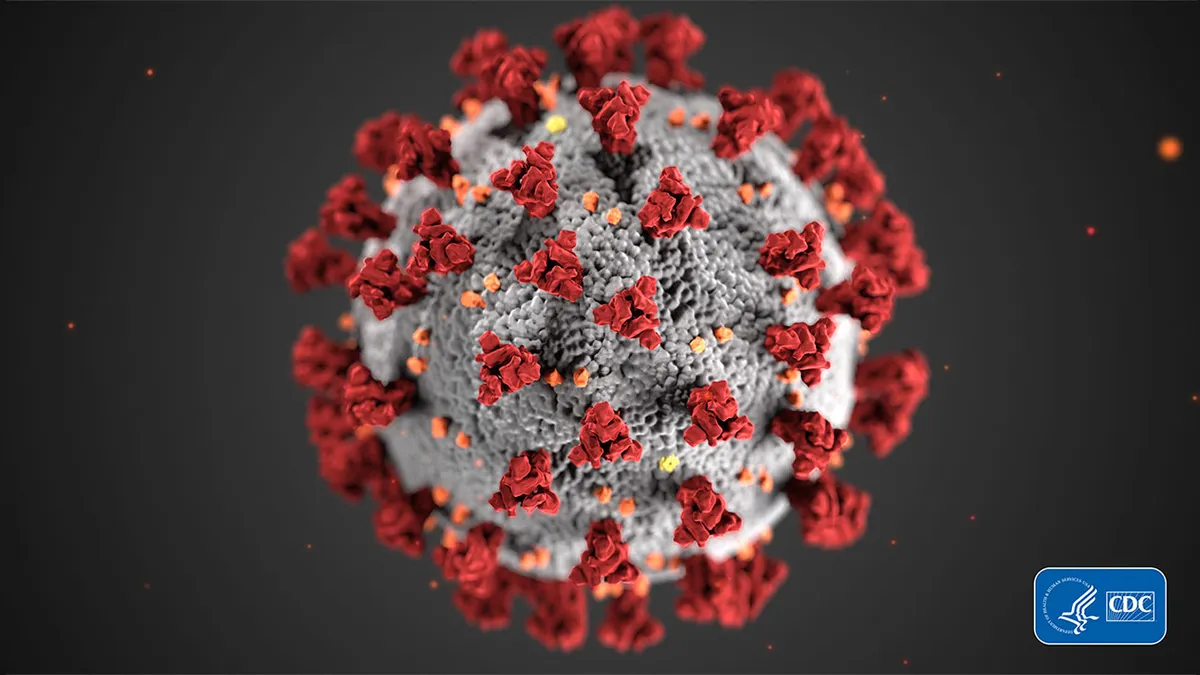
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
ACC 2020 meeting canceled as coronavirus spreads in US
Abbott, Edwards and Medtronic were among the medtechs slated to present data at the American College of Cardiology's annual event. "This is a unique time for us all," the organization wrote in a statement Monday.
By Maria Rachal • March 9, 2020 -

 CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on black background". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
$8.3B in coronavirus funding set in motion as federal agencies ramp up response
The House and Senate passed a bill with allocations for medical supplies and testing as Vice President Mike Pence met with clinical lab execs Wednesday from LabCorp, Quest Diagnostics, Thermo Fisher and others.
By Shannon Muchmore • Updated March 5, 2020 -
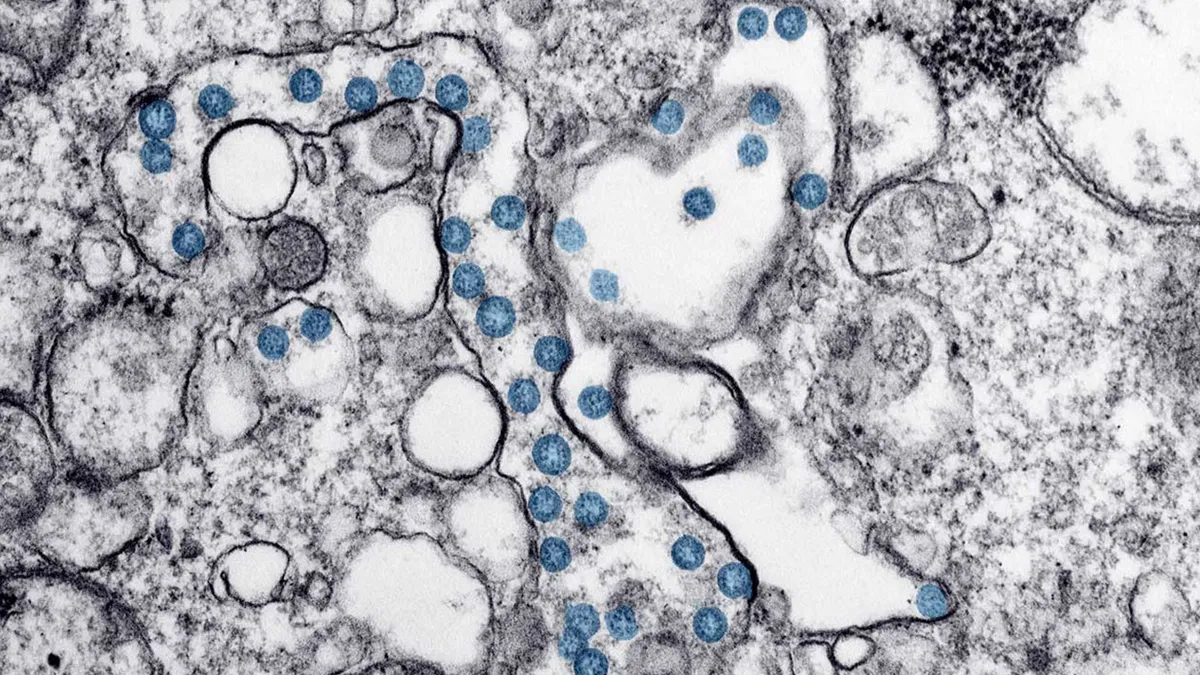
 CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Hannah A Bullock; Azaibi Tamin. (2019). "covid-19 coronavirus microscopic image with blue colored viral particles". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
LabCorp launches coronavirus test, Quest in the wings
The updates from the major U.S. clinical labs come days after FDA OK'ed the use of lab developed tests that have not yet received emergency use authorization.
By Maria Rachal • March 5, 2020 -
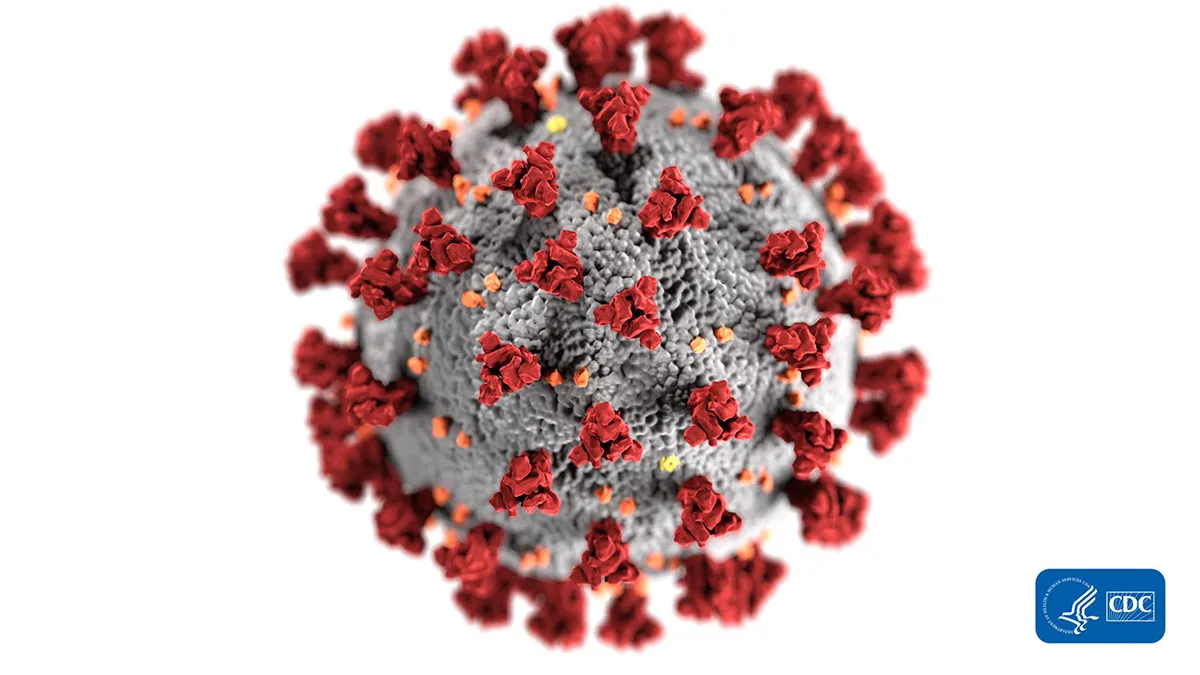
 CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
CDC/Alissa Eckert, MS. "covid-19 coronavirus on white". Retrieved from https://www.cdc.gov/media/subtopic/images.htm.
Insurer lobby urges coronavirus test coverage, but can't mandate changes
“They can’t require member companies to do anything,” said Sabrina Corlette, a research professor at Georgetown University's Center on Health Insurance Reforms.
By Samantha Liss • March 5, 2020 -
UK looks to use Brexit to make device industry more transparent
The House of Commons bill seeks to address a regulatory gap for medical devices, as the previous legal framework is based on EU directives. One element provides new information-sharing powers related to safety.
By Nick Paul Taylor • March 5, 2020 -
Exact Sciences closes takeovers of 2 cancer testing businesses
The deals give the diagnostics company a solid tumor test and R&D capabilities as it aims to expand beyond its flagship Cologuard product.
By Nick Paul Taylor • March 4, 2020 -
After waffling, Qiagen agrees to $11.5B buyout by Thermo Fisher
Months of will-they-won't-they M&A rumors culminated in the Dutch test maker accepting an offer for a 23% premium on its shares, as Thermo Fisher aims to strengthen its molecular diagnostics and infectious disease efforts.
By Maria Rachal • March 3, 2020 -
FDA seeks 'right balance' as it permits immediate use of coronavirus tests
"We are not changing our standards for issuing Emergency Use Authorizations," Commissioner Stephen Hahn said Saturday as the agency issued new guidance aimed at accelerating testing capacity in the U.S.
By Susan Kelly • March 2, 2020 -
Coronavirus: No US shortages of essential devices yet, as FDA monitors more than 60 companies
Still, manufacturers operating 72 plants in China face workforce pressures and surging demand for some equipment, FDA said in an update late Thursday.
By Nick Paul Taylor • Feb. 28, 2020 -
FDA pilots new 510(k) submission template for device manufacturers
The voluntary assessment will enroll up to nine participants that reflect the makeup of the medical device industry.
By Nick Paul Taylor • Feb. 27, 2020 -
FDA bolsters CLIA waiver application recommendations
The agency added new sections on failure alerts, labeling and safeguards, doubling the length of a guidance text for in vitro diagnostic manufacturers in the process.
By Nick Paul Taylor • Feb. 26, 2020 -
Guardant predicts net loss doubling as it takes on Exact Sciences' Cologuard
The cancer testing company's CEO said new studies could open up significant market opportunities, justifying the spending surge.
By Nick Paul Taylor • Feb. 25, 2020 -
1st test for genetic condition tied to developmental delay wins FDA nod
Austin, Texas-based biotech Asuragen won De Novo authorization for a blood test for Fragile X Syndrome, which the agency said is the most common known cause of inherited developmental delay and intellectual disability.
By Maria Rachal • Feb. 24, 2020 -
FDA tracks uptick in masks, other protective gear in coronavirus update
Commissioner Stephen Hahn said the shift in ordering patterns is yet to manifest in a shortage but warned Friday the situation is “evolving and very dynamic.”
By Nick Paul Taylor • Feb. 18, 2020





