Digital Health: Page 14
-
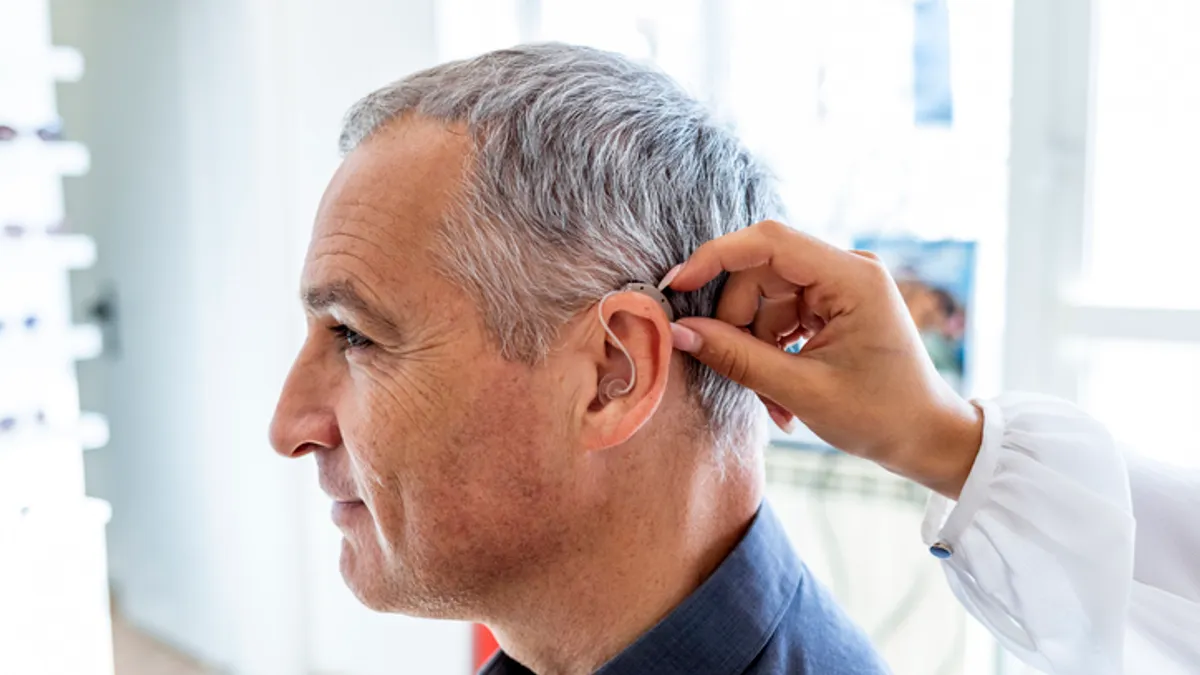
Lucid enters OTC hearing aid market, undercutting rivals with devices starting at $200
Lucid joins Sony and the Bose-partnered Lexie Hearing with aids priced thousands of dollars below prescription devices, putting auditory assistance within reach of tens of millions of Americans.
By Nick Paul Taylor • Oct. 18, 2022 -
Sponsored by Esper
Why Android is the future of connected HealthTech devices
How Android can make your connected HealthTech device strategy a success.
Oct. 17, 2022 -
Bose-partnered Lexie to launch $999 OTC hearing aid, challenging Sony, Eargo in nascent market
Lexie has matched the price of the first Sony device and is seeking to differentiate itself through the use of a rechargeable battery.
By Nick Paul Taylor • Oct. 14, 2022 -
AI matches humans at detecting mental health red flags in text messages, study shows
The study suggests algorithms have the potential to enable automated tools for clinical support of people with mental illness.
By Nick Paul Taylor • Oct. 13, 2022 -
‘Do-it-yourself’ artificial pancreas system beats production-line pump in controlled trial
Type 1 diabetes patients who used a customizable, open-source artificial pancreas, an insulin pump and a Dexcom G6 CGM found it more effective at controlling blood glucose than conventional sensor-augmented insulin pumps.
By Nick Paul Taylor • Oct. 12, 2022 -
Software to predict risk of sepsis, stroke should be regulated as a medical device, says FDA
Clarity on rules welcomed by some device makers, who also cautioned that products may take longer to come to market.
By Elise Reuter • Oct. 11, 2022 -
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott’s FreeStyle Libre 2 CGM beats fingerstick testing in independent clinical trial
Going into the study, the researchers were unsure if CGMs with optional alarms for high and low blood glucose levels benefit patients with Type 1 diabetes.
By Nick Paul Taylor • Oct. 11, 2022 -
Sponsored by Medrio
4 proven participant education techniques to improve electronic collection of informed consent
Patient education is vital to informed consent. Improve your approach with these best practices.
By Nicole Latimer, Chief Executive Officer • Oct. 10, 2022 -
NovaSight’s digital treatment for lazy eye gets FDA nod, providing alternative to patching
A randomized controlled trial found the digital device to be as effective as wearing an eye patch, the current gold standard for treatment, opening the way for more children to complete necessary therapy for amblyopia.
By Nick Paul Taylor • Oct. 10, 2022 -
Owlet seeks clearance of blood-oxygen-measuring baby sock after FDA warning letter
Owlet now is a step closer to selling a prescription medical device designed to alert parents when their baby’s heart rate or oxygen saturation levels move outside of prescribed ranges.
By Nick Paul Taylor • Oct. 10, 2022 -
Q&A
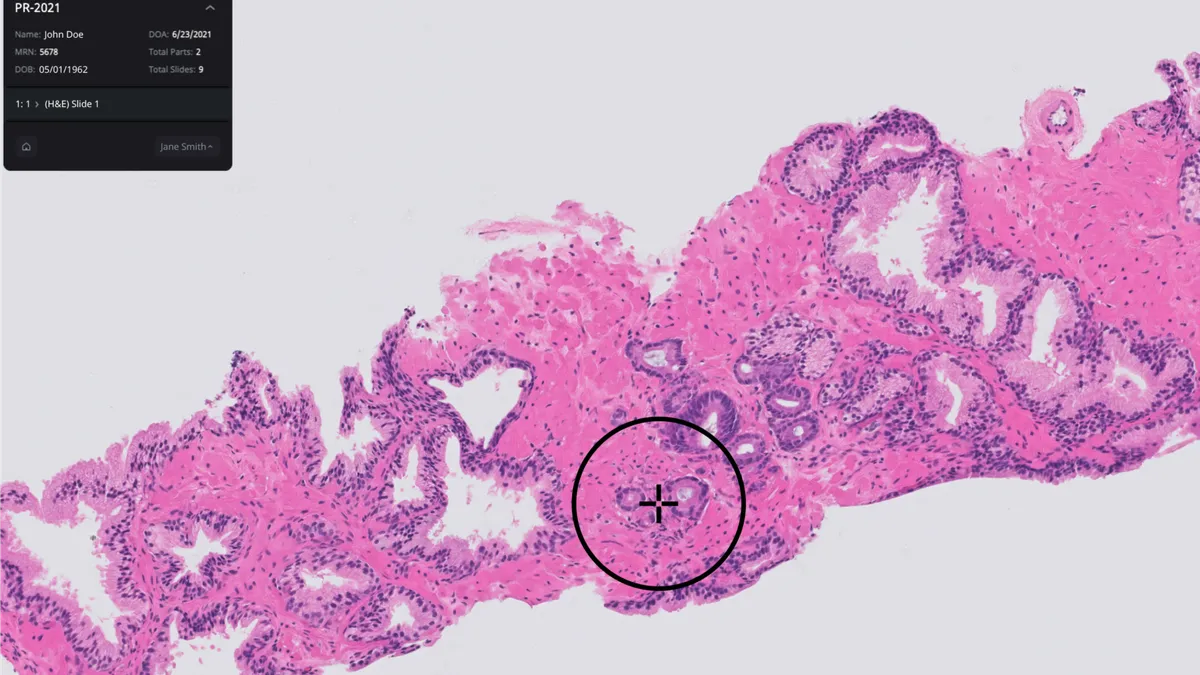
Friday Q&A: Paige CEO Andy Moye on how digital pathology is changing the diagnostic game
Commercial laboratories and academic medical centers are starting to adopt digital tools to help pathologists detect cancer, opening a large market for AI-powered diagnostic tools.
By Elise Reuter • Updated Oct. 8, 2022 -

 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Dexcom, Abbott have ‘massive opportunity’ with new CGM coverage proposal: analysts
Analysts at J.P. Morgan said the proposal reads “very favorably” for the two leading makers of continuous glucose monitors and unlocks “a major near-term driver for growth.”
By Nick Paul Taylor • Oct. 7, 2022 -
Dexcom starts global rollout of G7 CGM system, launching device in U.K. and Germany
Software updates have delayed the introduction of the product in the U.S. at a time when the company seeks to take market share from rival Abbott.
By Nick Paul Taylor • Oct. 6, 2022 -
Genentech, Winterlight track changes in Alzheimer’s using automated speech-based system
The researchers called the system a “promising prototype,” while cautioning that further validation is needed.
By Nick Paul Taylor • Oct. 5, 2022 -
Digital health funding collapsed in Q3, falling to lowest level since 2019: analysts
Analysts at Rock Health said “the market isn’t the same as it was” after seeing a sharp drop in late-stage deals.
By Nick Paul Taylor • Oct. 5, 2022 -
Medical machine-learning studies lack high-quality clinical trials, review shows
The findings “highlight areas of concern regarding the quality of medical machine learning RCTs and suggest opportunities to improve reporting.”
By Nick Paul Taylor • Oct. 4, 2022 -
European AI Act could have ‘significant impact’ on manufacturers, medtech group warns
“Overregulation and misalignment” could create uncertainty and stop products from coming to market, MedTech Europe says.
By Nick Paul Taylor • Oct. 3, 2022 -
Digital pathology gains momentum among US providers, vendors: report
Already popular in Europe, digital pathology technologies are primed for growth in the U.S., according to a report from KLAS research.
By Hailey Mensik • Sept. 30, 2022 -
Q&A
Friday Q&A: iRhythm CEO Quentin Blackford on how the heart monitor maker plans to reach $1B in sales
Continuing the company’s success in the U.S. and expanding internationally are the two biggest factors to achieving its $1 billion forecast, says Blackford.
By Ricky Zipp • Sept. 30, 2022 -
Bionic pancreas led to lower blood sugar levels than standard of care: study
The results point to the potential for a simplified approach for automated insulin delivery that could be particularly useful for patients without regular access to an endocrinologist.
By Elise Reuter • Sept. 29, 2022 -
FDA finalizes guidance on how clinical decision support software is regulated
The FDA has overhauled the 2019 draft, deleting some sections and completely rewriting other parts of the document.
By Nick Paul Taylor • Sept. 28, 2022 -
FDA makes case for ‘new regulatory paradigm’ amid hurdles in software-oversight program
The agency seeks to tailor requirements based on the latest science, the benefits and risks posed by devices, their real-world performance and their contribution to promoting health equity.
By Nick Paul Taylor • Sept. 27, 2022 -
Forget oximeters, smartphone cameras detect 79% of cases of low blood oxygen in small study
If the results are validated by wider studies, physicians will have a new tool to increase the number of people who can be monitored for chronic and acute respiratory conditions.
By Nick Paul Taylor • Sept. 23, 2022 -
Q&A
Friday Q&A: Scripps’ Jay Pandit talks about wearables, decentralized trials and what’s next for digital health
As wearables confirm their ability to collect data, the big question is what to do with it all. And the next technical challenge: improving wrist-based blood pressure monitors.
By Elise Reuter • Sept. 22, 2022 -

 Courtesy of https://www.dbl-diabetes.com/wp-content/uploads/2022/03/1114x708-dblg1-system-overview-EN.jpg.webp
Courtesy of https://www.dbl-diabetes.com/wp-content/uploads/2022/03/1114x708-dblg1-system-overview-EN.jpg.webp
Diabeloop’s insulin algorithm shows 17-point rise in time-in-range from year-long patient study
About half of the people using its device achieve the recommended outcomes, versus 27% of those using standard treatment.
By Nick Paul Taylor • Sept. 22, 2022