Medical Devices
-
Quantum Surgical buys J&J’s NeuWave
Quantum, developer of the Epione robotic platform for cancer treatment, said the acquisition supports its strategy of reducing cancer mortality through robot-assisted tumor ablation.
By Susan Kelly • Feb. 25, 2026 -
AdvaMed names Jake Leach as chair of diabetes sector
The Dexcom CEO will lead the lobbying group’s sector representing diabetes tech companies around the world.
By Elise Reuter • Feb. 24, 2026 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
Medtronic’s MiniMed prices IPO at up to $784M
The diabetes spinoff plans to offer 28 million shares at $25 to $28 each.
By Elise Reuter • Feb. 24, 2026 -
AdvaMed urges tailored tariff approach for medtech
CEO Scott Whitaker said the medtech lobbying group stands ready to work with President Donald Trump to address trade imbalances by confronting unfair foreign practices in the sector.
By Susan Kelly • Feb. 24, 2026 -
Medtronic to cut 81 employees at California diabetes site
As Medtronic prepares to spin out its diabetes business, a spokesperson said the company is considering the structure, capabilities and scale needed to succeed.
By Elise Reuter • Feb. 20, 2026 -
Medtronic’s blood pressure device wins coverage nod in Japan
Medtronic is investing in the rollout of the Symplicity Spyral renal denervation procedure, including hiring for new market development roles, CEO Geoff Martha said on an earnings call.
By Susan Kelly • Feb. 20, 2026 -
Senseonics launches automated insulin dosing system with 1-year CGM
The company has integrated its Eversense 365 implant with Sequel Med Tech’s insulin delivery system to automate the management of Type 1 diabetes.
By Nick Paul Taylor • Feb. 20, 2026 -
FDA issues early alert on Trividia glucometer issue linked to 114 injuries
Users may receive an error code either due to a test strip error or a very high blood glucose event, Trividia Health said.
By Elise Reuter • Feb. 19, 2026 -
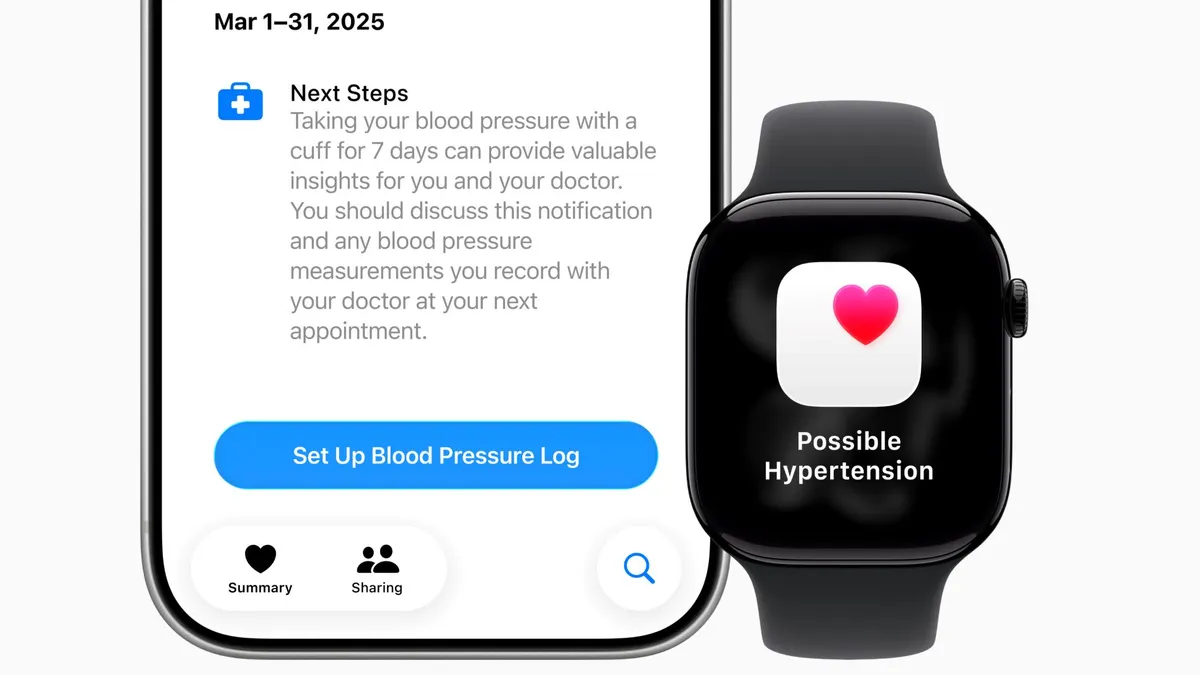
Apple Watch’s hypertension feature may miss some cases, researchers find
The absence of an alert provides limited reassurance, particularly for older adults or people with an elevated risk of high blood pressure, according to the study’s authors.
By Elise Reuter • Feb. 19, 2026 -
Medline addresses bed fire risk linked to death
The FDA said hand pendants and cords may overheat, causing the devices to spark, burn, melt, smoke and catch fire.
By Nick Paul Taylor • Feb. 18, 2026 -
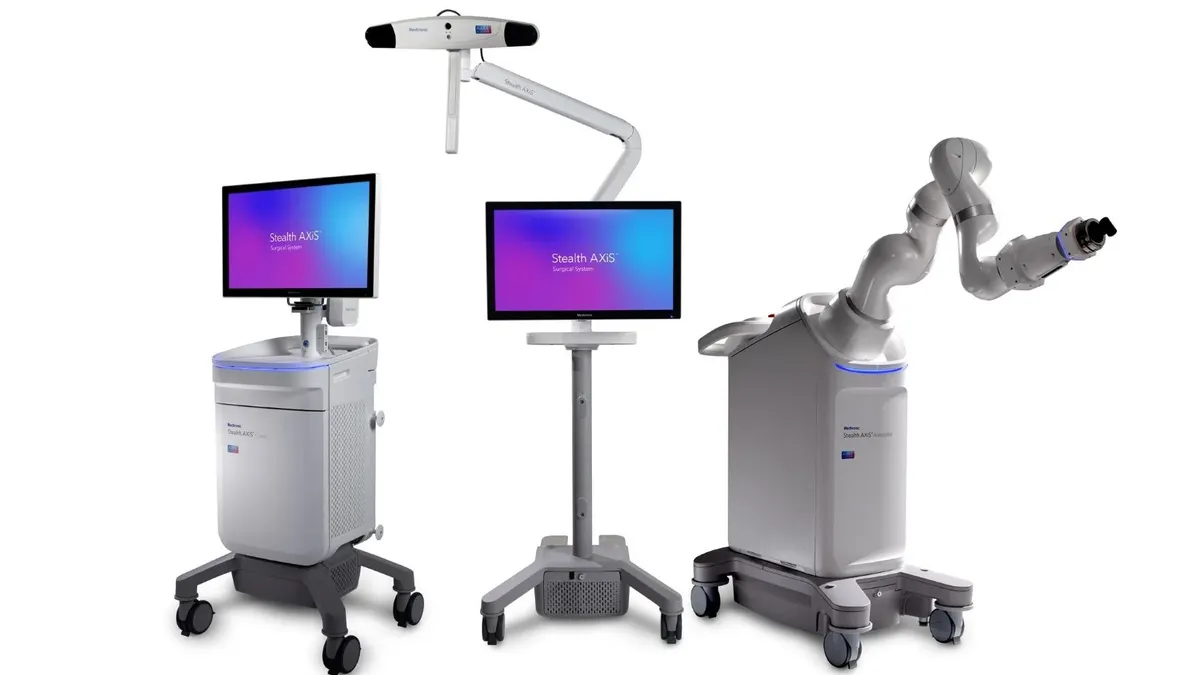
Medtronic nets FDA clearance for robotic spine system
Called Stealth AXiS, the surgical system builds on Medtronic’s Mazor robotic platform, as the medical device maker takes market share in the $15 billion cranial and spinal technologies sector.
By Susan Kelly • Feb. 18, 2026 -
Danaher to buy Masimo for $9.9B to boost diagnostics portfolio
Masimo’s board considered other potential partners before determining Danaher was an “ideal fit,” executives at the patient monitoring company said.
By Susan Kelly • Feb. 17, 2026 -
Dexcom seeks expanded Medicare coverage of CGMs for Type 2 diabetes
New CEO Jake Leach said the company plans a trial readout in mid-2026 to support coverage of its glucose sensors in Type 2 users who don’t take insulin.
By Elise Reuter • Feb. 13, 2026 -
Siemens Healthineers, Mayo Clinic partner on neurodegenerative disease, cancer
An expanded collaboration between the two organizations will focus on prostate cancer and metastatic liver tumors, in addition to certain neurological disorders.
By Susan Kelly • Feb. 13, 2026 -
Novocure wins FDA approval to treat pancreatic cancer with electric fields
Winning approval to target the 15,000 U.S. patients with locally advanced pancreatic cancer is the first step in a broader expansion in the tumor type.
By Nick Paul Taylor • Feb. 13, 2026 -
Neurent raises $74M to commercialize treatment for nasal condition
The device creates lesions to disrupt nerve signals that drive mucus production.
By Nick Paul Taylor • Feb. 12, 2026 -
AdvaMed names Melissa Torres as new leader for technology and regulatory affairs
Torres joins the medtech lobby from the FDA’s Center for Devices and Radiological Health, where she was associate director for international affairs.
By Susan Kelly • Feb. 12, 2026 -
Zimmer plans US salesforce reorganization
Zimmer Biomet expects the shift of its U.S. salesforce to a more specialized, direct approach will help it better compete in the orthopedics market.
By Elise Reuter • Feb. 11, 2026 -
Edwards focuses on earlier TAVR adoption
The heart valve specialist reported a second straight quarter of double-digit TAVR sales growth and credited recent study results with driving a “sense of urgency” for treating patients.
By Susan Kelly • Feb. 11, 2026 -
Baxter cuts roughly 90 jobs at IV solutions plant that recovered from Hurricane Helene
Hospital demand for IV solutions in the U.S. is lower than before Hurricane Helene, the company said.
By Elise Reuter • Feb. 10, 2026 -
4 takeaways from the 2026 AF Symposium
Johnson & Johnson, Boston Scientific and Abbott were among the companies showcasing new data on atrial fibrillation treatments, including pulsed field ablation catheters.
By Susan Kelly • Feb. 10, 2026 -
Stryker starts limited release of Mako RPS handheld knee robot
Surgeons interested in robotic technology but wanting a simpler system than existing platforms are the target market for the new device.
By Nick Paul Taylor • Feb. 10, 2026 -
Abbott details Volt, TactiFlex data; Pulse Biosciences’ results shine
Abbott and Pulse released their findings at the AF Symposium that wrapped this weekend in Boston.
By Susan Kelly • Feb. 9, 2026 -
FDA breakthrough program starts FY2026 at steady pace
Orthopedics was the most active area over the second half of 2025, with the FDA issuing 13 breakthrough designations to devices in the therapeutic area.
By Nick Paul Taylor • Feb. 9, 2026 -
J&J recalls coil systems used in aneurysm treatment
Healthcare providers are advised to stop using the devices after a failure to detach was linked with four serious injuries and one death.
By Susan Kelly • Feb. 6, 2026