Medical Devices: Page 105
-
Medtronic misses bid for early FDA OK in renal denervation, stock slides
The independent data safety monitoring board found the clinical trial needs to keep enrolling patients and gather results to show if the treatment works.
By Nick Paul Taylor • Oct. 18, 2021 -
Deep Dive
Anatomy of a medical device recall: How defective products can slip through an outdated system
"It truly is like we are operating in about the 1950s," one consultant noted of the process. The FDA has held two meetings in the past year to mull improvements.
By Ricky Zipp • Oct. 18, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtechs need strategy to prevent bias in AI-machine learning-based devices: FDA
The agency is mulling the types of information medtechs might include in labeling for such devices to support transparency. A Philips rep cautioned about the pitfalls of "information overload."
By Greg Slabodkin • Oct. 15, 2021 -
Biden circling former FDA chief Califf to again lead agency: report
The Washington Post reported the administration was "closing in" on the choice, citing unnamed sources. Still, the White House declined to comment and Califf could face opposition from Senate Democrats.
By Ricky Zipp • Oct. 15, 2021 -
Medtronic pledges R&D boost, brand refresh, amid rising Big Tech rivalry
CEO Geoff Martha said the device giant is still "building out that direct-to-consumer muscle" and acknowledged "healthy competition" with the likes of Apple and Google.
By Greg Slabodkin • Oct. 14, 2021 -
EU proposes to delay IVD Medical Device Regulation, citing COVID-19 backlog
Originally set to go into effect in May 2022, a progressive rollout is now planned. The proposal will now go to the European Parliament and Council for adoption, according to the announcement.
By Kim Dixon • Updated Oct. 14, 2021 -
Boston Scientific, Sterigenics potential targets of EPA ethylene oxide reporting requirements
The agency has written to the operators of 31 medical device sterilization facilities, outlining its plans and requesting information to inform its final decision.
By Nick Paul Taylor • Oct. 14, 2021 -
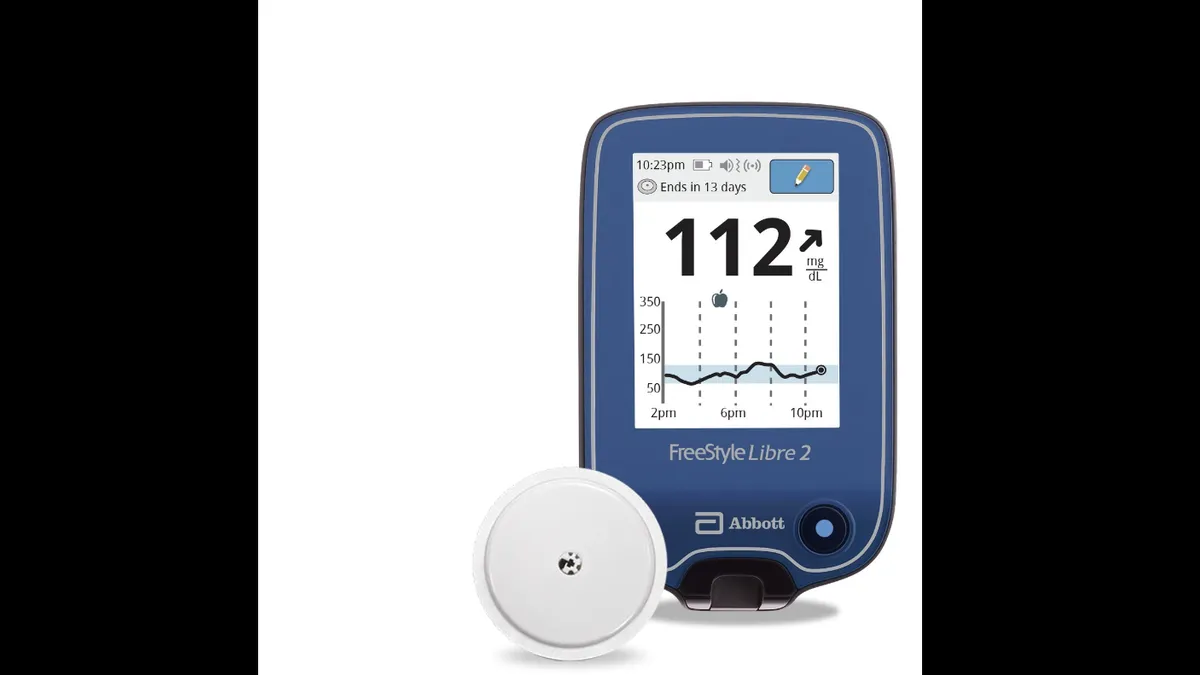
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott, Dexcom could benefit from CGM use jump among Type 2 diabetes patients: report
The population could drive the next wave of demand for devices to track and control diabetes, Jefferies analysts predict, based on polling of roughly 50 endocrinologists and general practitioners.
By Susan Kelly • Oct. 13, 2021 -
Withings gets FDA nod for AFib-detecting wearable, taking on Apple
The device, which is capable of detecting atrial fibrillation, combines an analog watch face with health features found in digital smartwatches. The French company joins Apple, Fitbit and Samsung in a crowded market.
By Nick Paul Taylor • Oct. 13, 2021 -
FDA breakthrough nods go to liquid biopsies for Alzheimer's, cancer drug response
The agency gave the designation to Quanterix's blood test, which has the potential to aid evaluation of people who present with cognitive impairment, and Nonagen Bioscience's non-invasive bladder cancer test.
By Nick Paul Taylor • Oct. 12, 2021 -
European Commission adds 2 modules to delayed Eudamed database, with no follow-on timeline
Sections on UDI/device registration, and Certificates and Notified Bodies are now available for voluntary use but some features are missing.
By Nick Paul Taylor • Oct. 12, 2021 -
Medtronic's surgical robot Hugo gains CE mark, setting up Intuitive faceoff
The system will hit the European market after the medtech giant unveiled it in 2019 and delayed the timeline amid the pandemic. Medtronic will take on market leader Intuitive Surgical and an upcoming product from J&J.
By Ricky Zipp • Oct. 11, 2021 -
4 takeaways from a bumper year of M&A medtech activity (so far)
After hunkering down last year, many companies entered 2021 with large cash reserves and acquisition targets that included players lacking scale to weather the downturn.
By Nick Paul Taylor • Oct. 11, 2021 -
FDA beefs up surgical stapler oversight after injuries, death reports
While medtechs like J&J and Medtronic backed the broad change, the final rule held firm on several contentious elements. The agency also updated labeling guidance of both staplers and staples due to patient safety risks.
By Nick Paul Taylor • Oct. 8, 2021 -
Deep Dive
Will a software bill of materials help or hurt medical device cybersecurity?
President Joe Biden's executive order calls for SBOMs, and the FDA wants to require premarket submissions to have an inventory of third-party device components. AdvaMed is concerned the data could be exploited by hackers.
By Greg Slabodkin • Oct. 7, 2021 -
Medtechs opened 2021 with a flurry of M&A and have not stopped spending since
With deals ranging from tuck-ins to multibillion-dollar takeovers, here's a roundup of the M&A spree so far this year after activity came to a near halt amid the unpredictability of 2020.
Oct. 7, 2021 -
Boston Scientific inks $1.75B deal for Baylis, biggest in string of M&A
SVB Leerink analysts wrote the acquisition will add needed support in electrophysiology and structural heart, two of the highest growth markets within cardiology. It's the medtech's fifth acquisition of 2021.
By Ricky Zipp • Oct. 6, 2021 -
EU shares guide to MDR's 22 rules for classifying medical devices
If a manufacturer and notified body dispute a classification, the case may be referred to the competent authority in the country where the company has its registered place of business.
By Nick Paul Taylor • Oct. 6, 2021 -
Medtronic expands 2 MiniMed insulin pump recalls on ring flaw, cyber risks
The recalls have hit the medtech giant at a time when competitors Insulet and Tandem are ramping up in the insulin pump market and amid flagging sales in its diabetes unit.
By Susan Kelly • Oct. 5, 2021 -
FDA resists industry push to nix De Novo inspections in final rule
"The most controversial aspect was FDA's provision for ... an FDA inspection for what is tantamount to a 510(k)," noted Bradley Merrill Thompson, attorney at the law firm Epstein Becker Green.
By Nick Paul Taylor • Oct. 5, 2021 -
FDA's real-world evidence push hampered by data challenges, 'million-dollar question'
While the agency wants to tap information like electronic health records and wearables to make pre- and postmarket decisions, these sources do not have the same quality controls as clinical trials.
By Greg Slabodkin • Oct. 4, 2021 -
FDA hits nearly all MDUFA IV commitments despite pandemic disruptions
The main black mark in an audit was the failure to publish draft guidance on content for premarket submissions for software in a medical device.
By Nick Paul Taylor • Oct. 4, 2021 -

 Retrieved from AdvaMed on October 04, 2021
Retrieved from AdvaMed on October 04, 2021
MCIT, cyber, RWE and 3 more takeaways from AdvaMed's 2021 conference
The medtech industry gathered virtually and in person for the lobby's annual conference, with topics ranging from the kill-off of the breakthrough device payment pathway to the pandemic upending CDRH's 2021 reset.
Oct. 4, 2021 -
Ransomware attacks put availability of medical devices at risk: FDA cyber chief
Industry reached a "watershed moment" earlier this year when a device outage caused by malware endangered patient lives, Kevin Fu, acting director of cybersecurity at CDRH said. "That was something we haven't seen before."
By Greg Slabodkin • Oct. 1, 2021 -
AdvaMed defends J&J in $344M row with California court over mesh marketing
The lobby group filed an amicus curiae brief asking the appeals court to reverse the judgment against J&J, arguing it "undermines a manufacturer's ability to rely on FDA input when it comes to the adequacy of device labeling."
By Nick Paul Taylor • Oct. 1, 2021








