Medical Devices: Page 124
-
Top picks for who could run HHS, CMS under Biden
Former Obama adviser and ACA architect Ezekiel Emanuel as well as high-profile state officials like New Mexico Governor Michelle Lujan Grisham are among those considered likely advisers.
By Rebecca Pifer Parduhn • Nov. 10, 2020 -
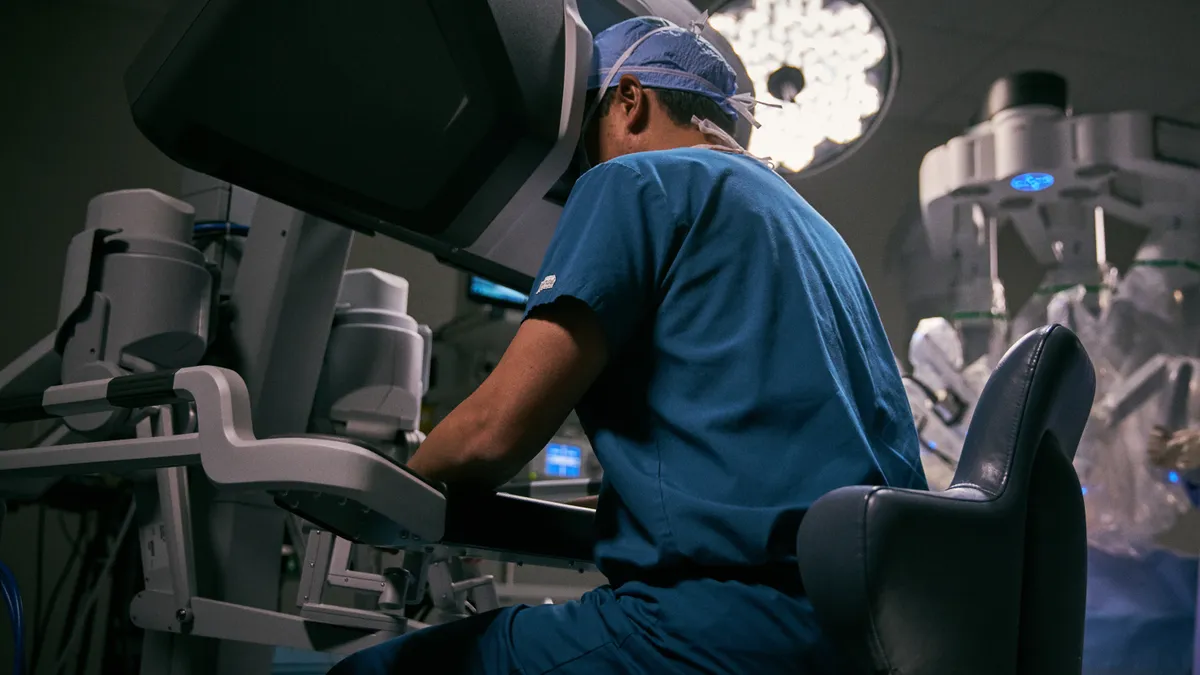
What Intuitive is looking for through its new $100M venture fund
Surgical robots have been Intuitive's sweet spot. As the company moves into VC for the first time, its broader focus on minimally invasive care, including interest in digital tools and precision diagnostics, opens more opportunities.
By Maria Rachal • Nov. 9, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Zimmer reports record robot installs, ASC-inspired M&A
While the company benefited from a rebound in elective procedures, execs warned investors the recovery slowed as the third quarter drew to a close.
By Nick Paul Taylor • Nov. 6, 2020 -
Insulet, Tandem raise 2020 outlooks as diabetes tech proves resilient to COVID-19 headwinds
Concerns that the pandemic would depress the number of new insulin pump customers have played out less severely than predicted.
By Maria Rachal • Nov. 6, 2020 -
Patients shirked diagnostic testing, in-person preventive care amid telehealth boom this spring
Routine preventive services that can't be done virtually, such as mammograms and colonoscopies, plunged 65% in March and April compared to the year prior, according to an analysis published in JAMA Network Open.
By Hailey Mensik • Nov. 5, 2020 -
Even if Biden wins, divided Congress stifles chance for more progressive health policies
Results of the election are not final and may be uncertain for weeks, but the most likely scenario points to mostly incremental change, a positive for many parts of the healthcare sector.
By Shannon Muchmore • Nov. 5, 2020 -
BD returns to growth on COVID-19 antigen tests, but predicts price erosion
The company said sales related to its rapid testing could hit $1.5 billion next year, with the prospect of demand continuing into 2022. However, management thinks Abbott's $5 test could drive down its pricing.
By Nick Paul Taylor • Nov. 5, 2020 -
Docs paid by ICD makers more likely to favor manufacturer: JAMA study
Yale researchers found patients were more likely to receive certain cardiac devices from the company that made the largest payment to their doctor, though quality of care did not appear to be affected.
By Susan Kelly • Nov. 4, 2020 -
Stryker closes Wright Medical acquisition after year-long wait
The Kalamazoo, Michigan-based medtech highlighted the shoulder, elbow, wrist, hand, foot and ankle devices it's gained, along with preoperative planning technology, now that the deal is final.
By Maria Rachal • Updated Nov. 11, 2020 -
MedPAC pans CMS idea to lean on commercial payers for Medicare coverage decisions
The advisory group warned against threats to transparency and rigor in response to a proposed rule defining the term "reasonable and necessary" and adding a faster coverage pathway for FDA-designated breakthrough devices.
By Susan Kelly • Nov. 3, 2020 -
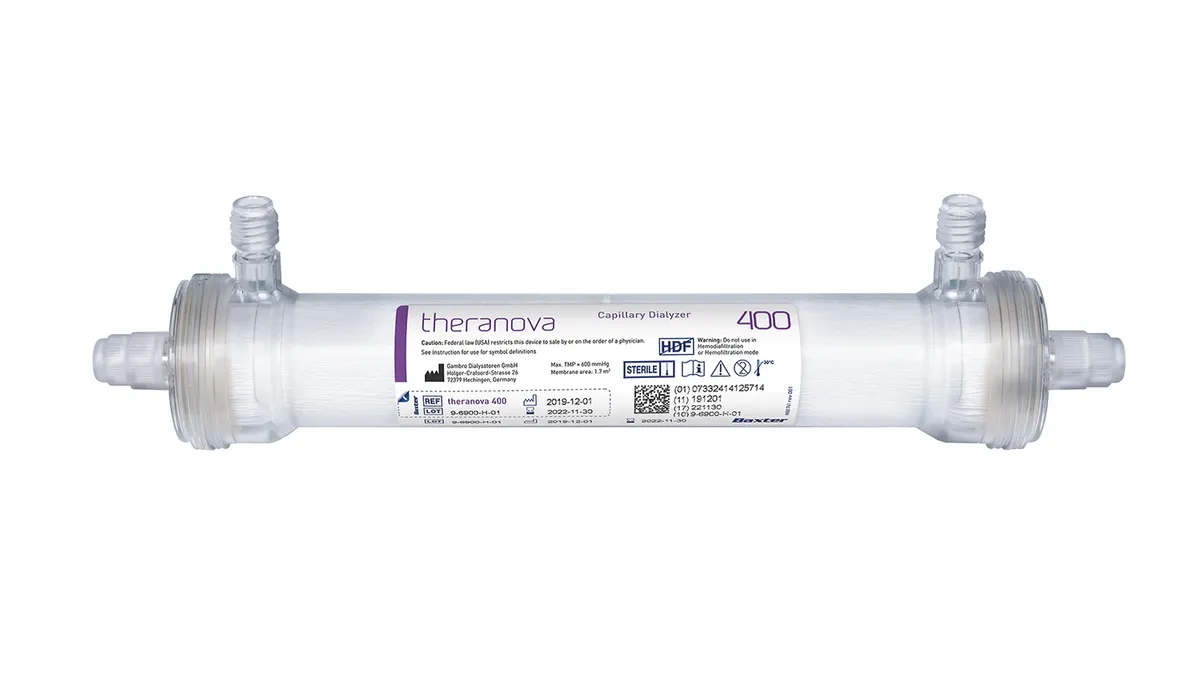
CMS finalizes rejection of Baxter, Outset dialysis device add-on payments
The broader, now final, ESRD rule is meant to support use of equipment and supplies for at-home dialysis treatment.
By Nick Paul Taylor • Updated Nov. 3, 2020 -
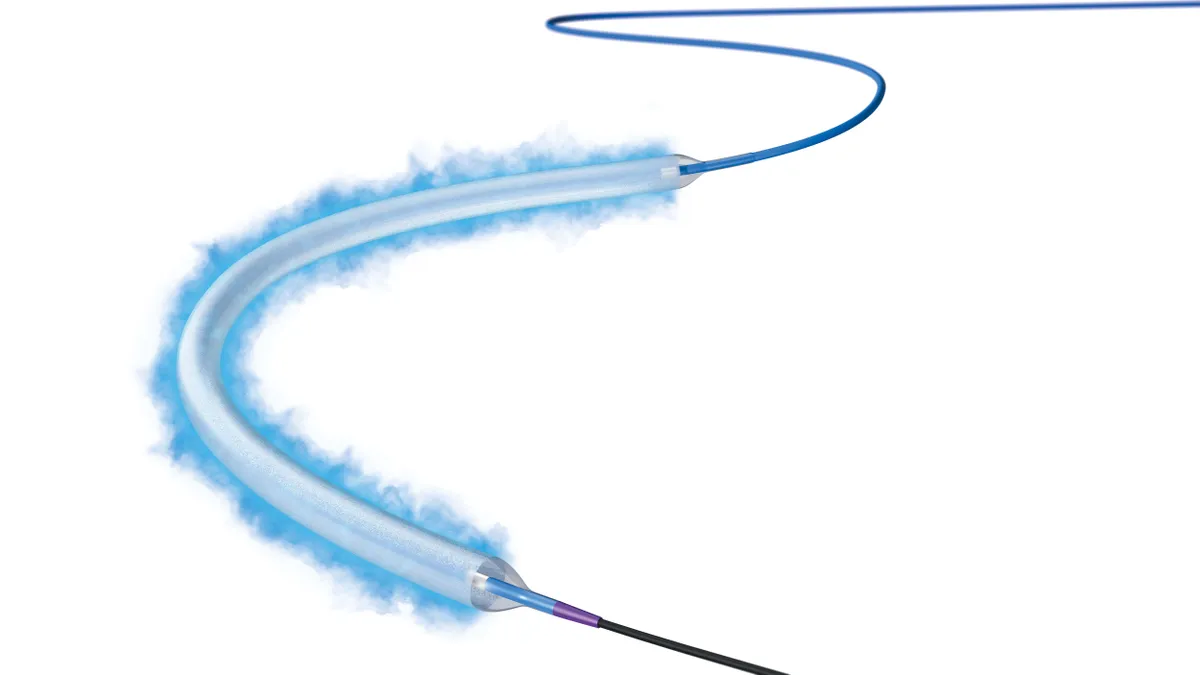
FDA approves Boston Scientific paclitaxel-coated balloon as sector revives after scrutiny
Studies have failed to confirm a late mortality signal that prompted an agency warning and advisory committee meeting in 2019. Boston Scientific now sees the recovery of the devices as a tailwind for its business.
By Nick Paul Taylor • Nov. 3, 2020 -
Stryker, NuVasive, Globus forge on with surgical robots through COVID-19
Despite worries that the pandemic's financial strain on customers may slow adoption of the expensive tools for knee, hip and spine surgery, device makers' Q3 reports reveal momentum.
By Maria Rachal • Oct. 30, 2020 -
Medtronic to pay $9.2M to settle DOJ influence, kickback allegations
The medtech giant is accused of offering "lavish" benefits in promoting a certain infusion pump to a South Dakota neurosurgeon, and misreporting those payments under a required CMS program.
By Nick Paul Taylor • Oct. 30, 2020 -
Medtronic acquires thyroid surgery tech in 7th tuck-in deal of 2020
An addition to the medtech's pandemic-battered ENT business, Ai Biomed's PTeye system won De Novo authorization from FDA in 2018 for its ability to help surgeons spare important calcium-regulating tissue during procedures.
By Maria Rachal • Oct. 29, 2020 -
Baxter Q3 sales up 4% as virus-driven demand offsets headwinds
The renal care business continues to grow during the pandemic but lower ER utilization and fewer hospitalizations, particularly in the U.S., hurt revenues by more than $65 million in the quarter.
By Greg Slabodkin • Oct. 29, 2020 -
Teleflex lines up $525M to buy hemostat specialist Z-Medica
The medtech predicts the trauma surgery and emergency medicine-focused products will begin adding up to $70 million in revenue next year, providing growth drivers outside its key interventional urology segment.
By Maria Rachal • Oct. 29, 2020 -
CMS competitive bidding process fails to drive expected savings, sparking rethink
While Needham analysts contend one action removes an overhang for Inogen, Invacare and ResMed, those at Jefferies see a "major negative" in the agency's determination to lower rates outlined in a proposed rule.
By Nick Paul Taylor • Oct. 29, 2020 -
Smith & Nephew lays path to 10% growth for acquired Integra assets
The medtech is moving deeper into the extremity orthopaedics turf fought over by the likes of Zimmer Biomet and Stryker acquisition target Wright Medical.
By Nick Paul Taylor • Oct. 29, 2020 -
Dexcom revenue growth dampened amid shift to lower pharmacy prices
The 26% rise in Q3 revenue, which came from a nearly 40% increase in sales, beat analysts' consensus estimates and were good enough for the company to raise its 2020 outlook. Still, shares closed down 9% on Tuesday.
By Maria Rachal • Oct. 28, 2020 -
Neovasc angina device fails to win FDA panel backing, stock tumbles 43%
By a vote of 17-1, experts on FDA's circulatory system devices panel said the available data did not provide reasonable assurance of effectiveness, though most thought the Reducer system was safe for patients.
By Susan Kelly • Oct. 28, 2020 -
Boston Scientific sales not yet back to growth as Watchman hit compounds COVID-19 impacts
The medtech is aiming for organic revenue growth in Q4 after falling 5.7% in Q3. CEO Mike Mahoney said estimating the remaining procedure backlog and new patient funnel "remain a challenge" and vary by business and region.
By Nick Paul Taylor • Oct. 28, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

MDUFA V talks kick off as FDA grapples with onslaught of COVID-19 submissions
Medtech industry groups broadly expressed a desire to maintain the status quo after FDA Commissioner Stephen Hahn described the strain on agency workers under MDUFA IV as unsustainable.
By Maria Rachal • Oct. 28, 2020 -
Neovasc refractory angina treatment faces FDA panel review
The agency's ultimate approval decision on the Canadian company's CE-marked device has important financial implications for the medtech, which saw its revenue plunge and operating loss deepen in the second quarter.
By Susan Kelly • Oct. 27, 2020 -
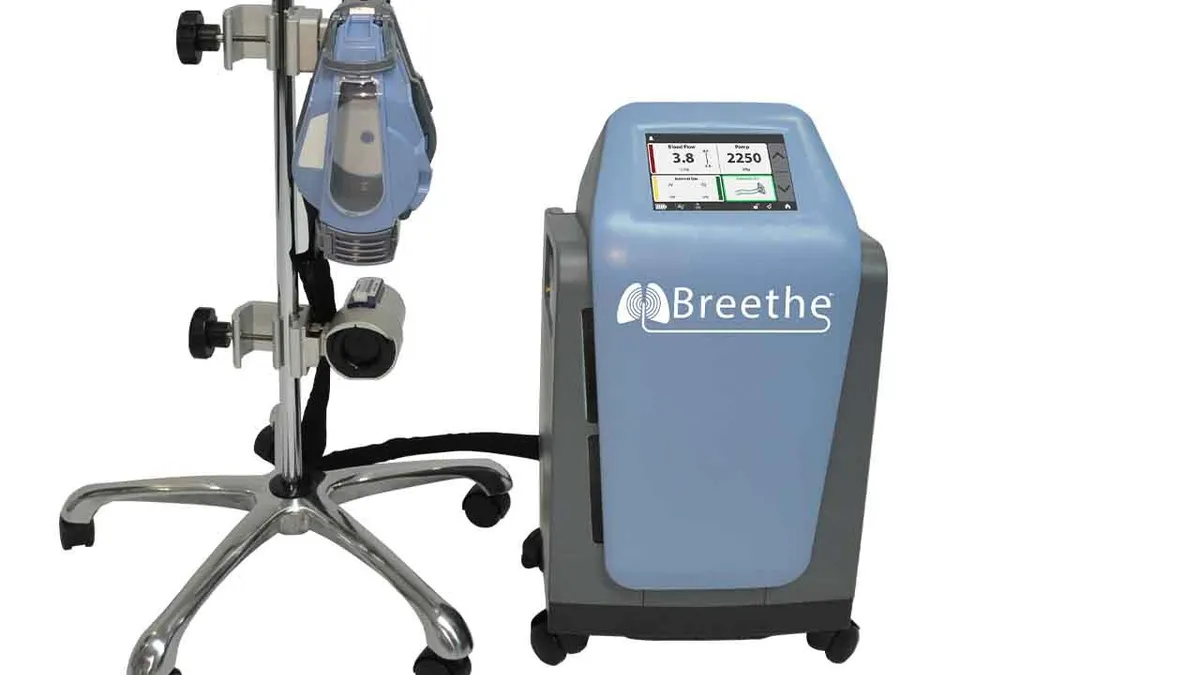
 Retrieved from Abiomed/BusinessWire on April 30, 2020
Retrieved from Abiomed/BusinessWire on April 30, 2020
FDA grants 510(k) to Abiomed's artificial lung, teeing up use in COVID-19
While LivaNova and Medtronic already sell ECMO devices, Abiomed is focusing on a relatively compact design to differentiate the device from the pack.
By Nick Paul Taylor • Oct. 27, 2020