Medical Devices: Page 163
-
Boston Scientific says Watchman more cost effective than drugs for AFib
In the Journal of the American Heart Association, researchers examined long-term data pooled from the Protect AF and Prevail clinical trials of the company's Watchman device for stroke prevention in atrial fibrillation patients.
By Susan Kelly • July 1, 2019 -
Trump: US won't impose tariffs on $300B Chinese goods 'for the time being'
Existing 25% tariffs on $250 billion of Chinese goods, along with China's retaliatory tariffs, will remain in place.
By Shefali Kapadia • June 29, 2019 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Interagency workshop to focus on device interoperability
The Health Information Technology Research and Development Interagency Working Group is seeking feedback from industry, academia and government experts on potential solutions for creating interoperable systems.
By Susan Kelly • June 28, 2019 -
Hologic promotes replacement to head diagnostics unit
Kevin Thornal, who is currently the president of the company's medical aesthetics division, will become the president of its diagnostics unit.
By Nick Paul Taylor • Updated July 22, 2019 -
Medtronic insulin pump recall lands in FDA's top risk category after cybersecurity warning
The Class I determination follows an FDA warning in June that a hacker could dangerously tamper with insulin dosing. As of Tuesday, the agency is unaware of any cases of patient harm stemming from the vulnerability.
By Nick Paul Taylor • Updated Nov. 5, 2019 -
Boston Scientific touts potential of BTG buy, structural heart strategy
The medtech giant said it plans to launch roughly 75 products and achieve up to 9% organic revenue growth by 2022, not including contributions to its interventional oncology business from the yet-to-be-finalized BTG acquisition.
By Maria Rachal • June 27, 2019 -
Health Canada to mandate medical device adverse event reporting
The new regulations will force hospitals to report incidents within 30 days.
By David Lim • June 27, 2019 -
Anthem adds Teleflex's UroLift to prostate disease coverage
The benign prostatic hyperplasia update will provide 32 million people insured by Anthem access to the implant.
By Nick Paul Taylor • June 27, 2019 -
FDA finalizes guidance on diagnostic ultrasound systems
The text hews closely to the draft the agency issued in 2017, the core of which detailed its policy on 510(k) filings.
By Nick Paul Taylor • June 27, 2019 -
US medtech market forecast to top $200B by 2023
The prediction from Fitch Solutions is based on trends like an aging population with a rising incidence of chronic disease and certain regulatory changes.
By Nick Paul Taylor • June 26, 2019 -
Smartphone heart data mirrors gold standard EKG in study
UCSF researchers said it was the largest analysis of real-world heart rate data remotely obtained and stratified by demographics and medical conditions.
By Nick Paul Taylor • June 26, 2019 -
Deep Dive
Looming EPA ethylene oxide rules hang over device supply chain
The medical device industry is lobbying Capitol Hill and statehouses, warning limited access to the sterilant could pose deadly disruptions.
By David Lim • June 25, 2019 -
Mayo Clinic to build proton therapy facility on Florida campus
A spokesperson for the health system said Mayo has not yet decided which manufacturer's proton therapy systems will outfit the planned center, set to be completed in late 2023.
By Susan Kelly • June 25, 2019 -
Teleflex recalls more endotracheal tubes, now linked to four deaths
The devices, used to administer anesthesia and provide ventilation during surgical procedures, may become disconnected from the breathing circuit, resulting in insufficient oxygenation.
By Susan Kelly • Updated Aug. 5, 2019 -
Final TAVR Medicare decision gets thumbs up from Edwards, Medtronic
The new policy eases volume requirements for hospitals and physicians to begin transcatheter aortic valve replacement programs but increases the number of valve procedures necessary to maintain a program.
By Susan Kelly • June 24, 2019 -
FDA ends alternative reporting program, pledges to make MAUDE user friendly
The agency disclosed more than 6 million medical device adverse event reports were filed under the controversial system since 1997.
By David Lim • June 24, 2019 -
Label should warn patients of paclitaxel device mortality signal, FDA panel says
The advisory committee struggled through the two-day meeting evaluating the safety of the devices due to incomplete data. Members agreed more data is needed but differed on the method the agency should use to collect it.
By David Lim • June 21, 2019 -
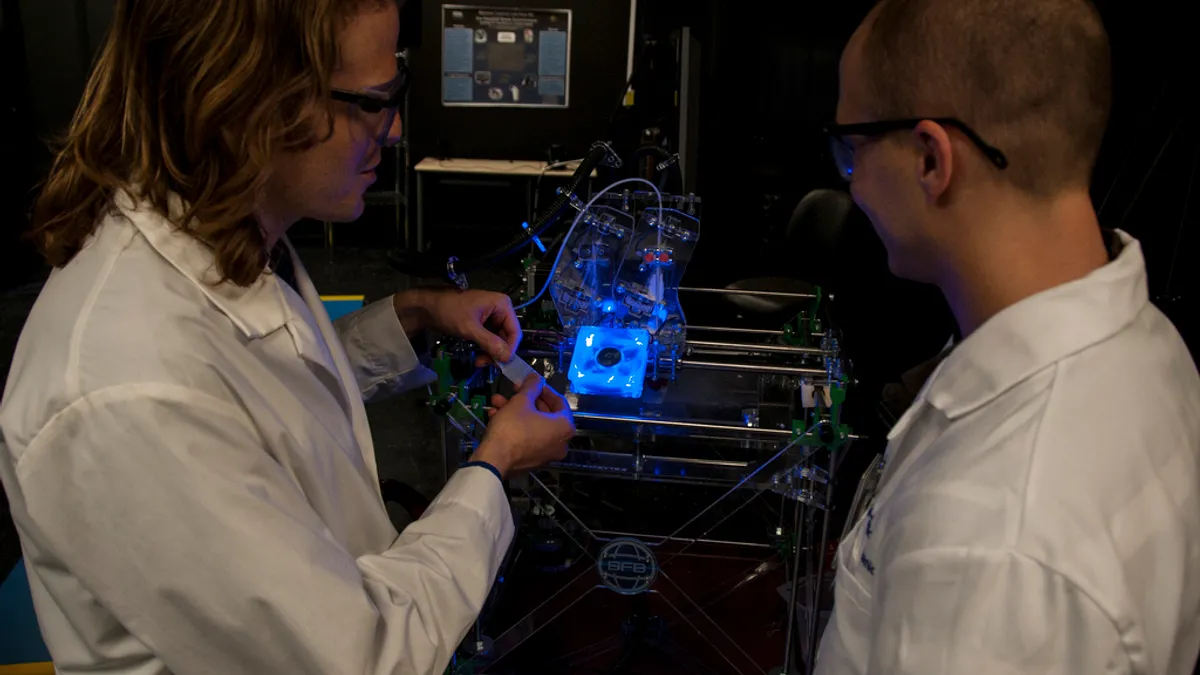
NIH's Collins highlights progress on 3D printed human organs
Researchers at Rice University found a way to create an open-source bioprinting technology that grows soft hydrogel scaffolds one layer at a time.
By Susan Kelly • June 21, 2019 -
Glaukos strikes deal to buy retinal drug delivery company
The takeover will give Glaukos ownership of micro-invasive, sustained-released, bio-erodible drug delivery platforms.
By Nick Paul Taylor • June 21, 2019 -
SEC ends Misonix foreign corruption probe without action
The maker of ultrasonic devices triggered the investigation in 2016 when it contacted the SEC with information about the business practices of its Chinese distributor.
By Nick Paul Taylor • June 21, 2019 -
FDA safety panel pans lack of paclitaxel device postmarket data on Day 1
The agency's Circulatory System Devices Panel Advisory Committee agreed Wednesday there is a mortality signal associated with paclitaxel-coated balloons and paclitaxel-eluting stents.
By David Lim • June 20, 2019 -
Philips gets FDA approval for over-the-counter defibrillators
The devices were previously cleared for sale in the U.S. via the 510(k) pathway but now need PMA approval.
By Nick Paul Taylor • June 20, 2019 -
Bursting balloon catheters prompt Cook Medical recall
The action comes less than a year after the Bloomington, Indiana-based device maker resolved an FDA warning letter faulting its quality management system.
By Susan Kelly • June 19, 2019 -
Swiss notified body to exit sector, heightening EU capacity fears
QS Zürich’s withdrawal will leave Switzerland with one medtech notified body, as new rules for the bloc near.
By Nick Paul Taylor • June 19, 2019 -
FDA approves cochlear implant designed for MRI
The implant is designed for safe access to magnetic resonance imaging scans and doesn't require a head wrap or removal of the device's internal magnet.
By Susan Kelly • June 18, 2019