Medical Devices: Page 81
-

 Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Medtronic’s HVAD system recalled again amid driveline cover problems
New issues have continued to surface with the problem-plagued device even after Medtronic pulled it from the market last year.
By Elise Reuter • Dec. 1, 2022 -
GE’s board finalizes healthcare spinoff
The new company, GE Healthcare, will be the sixth largest global medtech company when it starts trading on Nasdaq on Jan. 4.
By Elise Reuter • Dec. 1, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Eargo raises $32M to fight rivals in over-the-counter hearing aid market
Investment firm takes majority stake, as Eargo faces competition from new rivals including Sony and Bose-partnered Lexie.
By Nick Paul Taylor • Dec. 1, 2022 -
European biomedical group backs delaying MDR certification deadline amid ‘looming crisis’
Pushing back the recertification deadline may buy time to address problems such as the shortage of capacity at certifying organizations.
By Nick Paul Taylor • Dec. 1, 2022 -
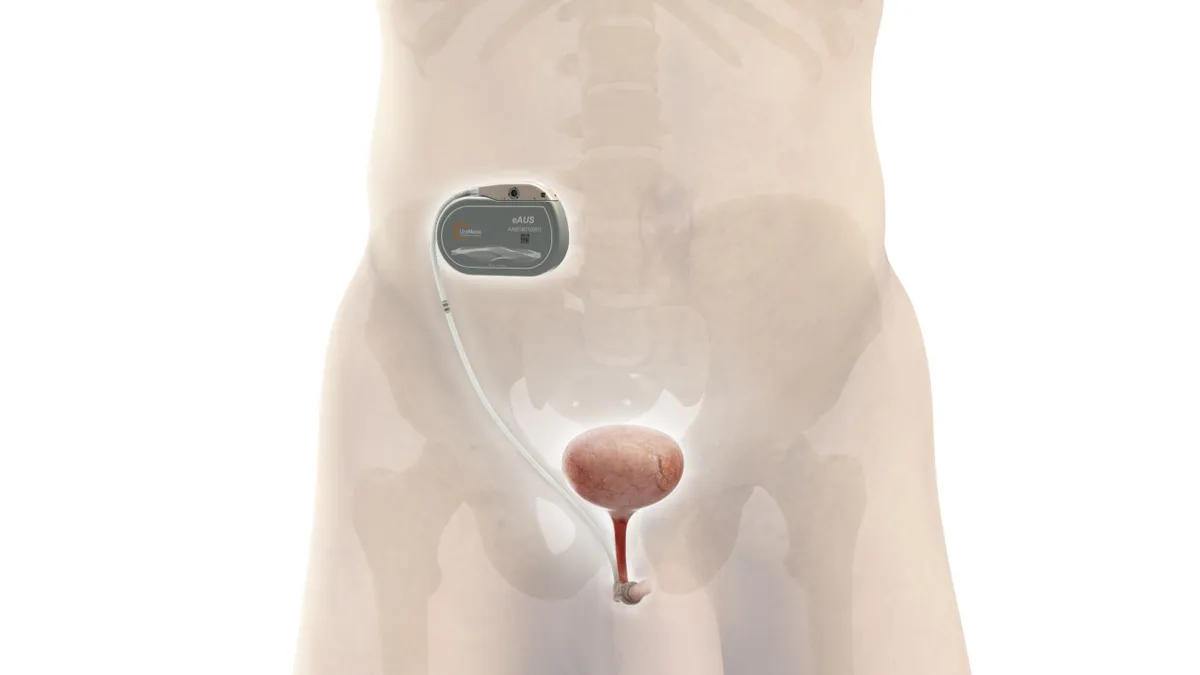
UroMems starts clinical trial of smart, automated implant to treat incontinence
The device is designed to improve on products such as Boston Scientific’s AMS 800 by eliminating the need for manual adjustments.
By Nick Paul Taylor • Nov. 30, 2022 -
Q&A
MedTech Innovators: Tissium co-founder Maria Pereira on taking tech from bench to bedside
The MIT-trained scientist explains how she went from PhD candidate to startup founder whose company is preparing to commercialize a range of products for use in reconstructing damaged tissue.
By Susan Kelly • Nov. 30, 2022 -
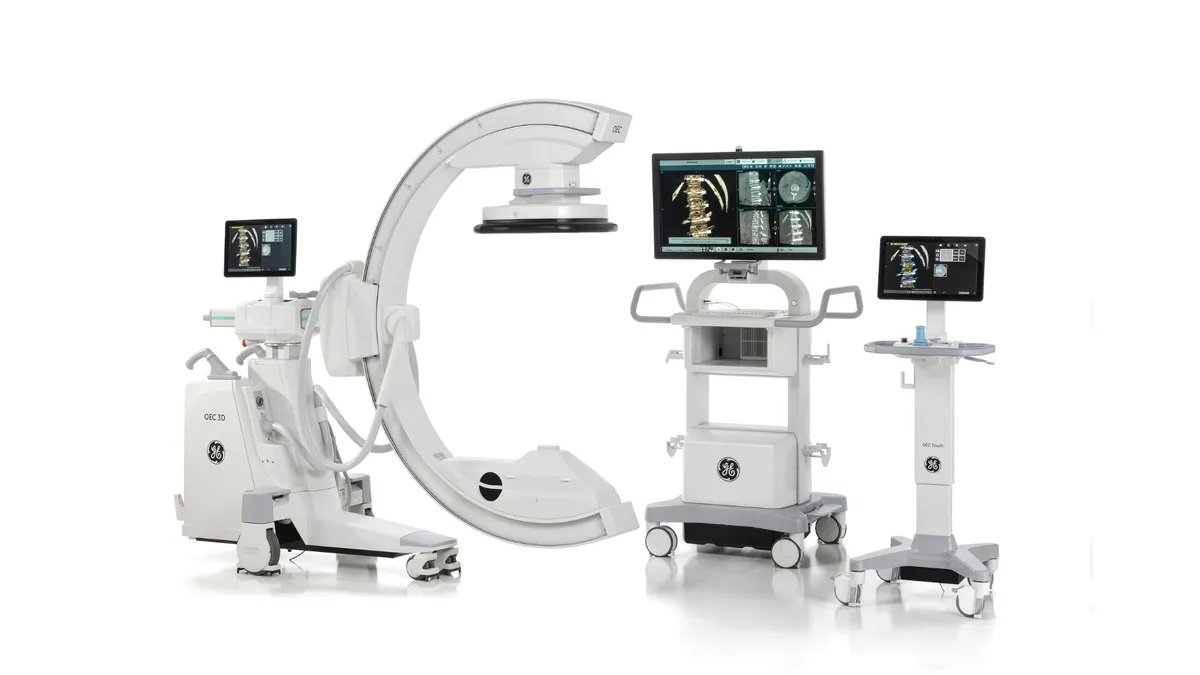
GE Healthcare, MediView XR partner to launch augmented reality X-ray imaging system
The companies are pitching the product as a response to physician demand for streamlined workflows and improved ergonomics.
By Nick Paul Taylor • Nov. 30, 2022 -
Medtronic touts benefits of latest Evolut TAVR system in retrospective analysis
Changes to the device improve alignment that is associated with improved coronary blood flow and valve hemodynamic performance.
By Nick Paul Taylor • Nov. 30, 2022 -
Boston Scientific to buy Apollo Endosurgery for $615M as entry into endobariatric market
The acquisition values Apollo shares at two-thirds above their closing price Monday, and may herald a new trend in medtech M&A, says an analyst.
By Elise Reuter • Nov. 29, 2022 -
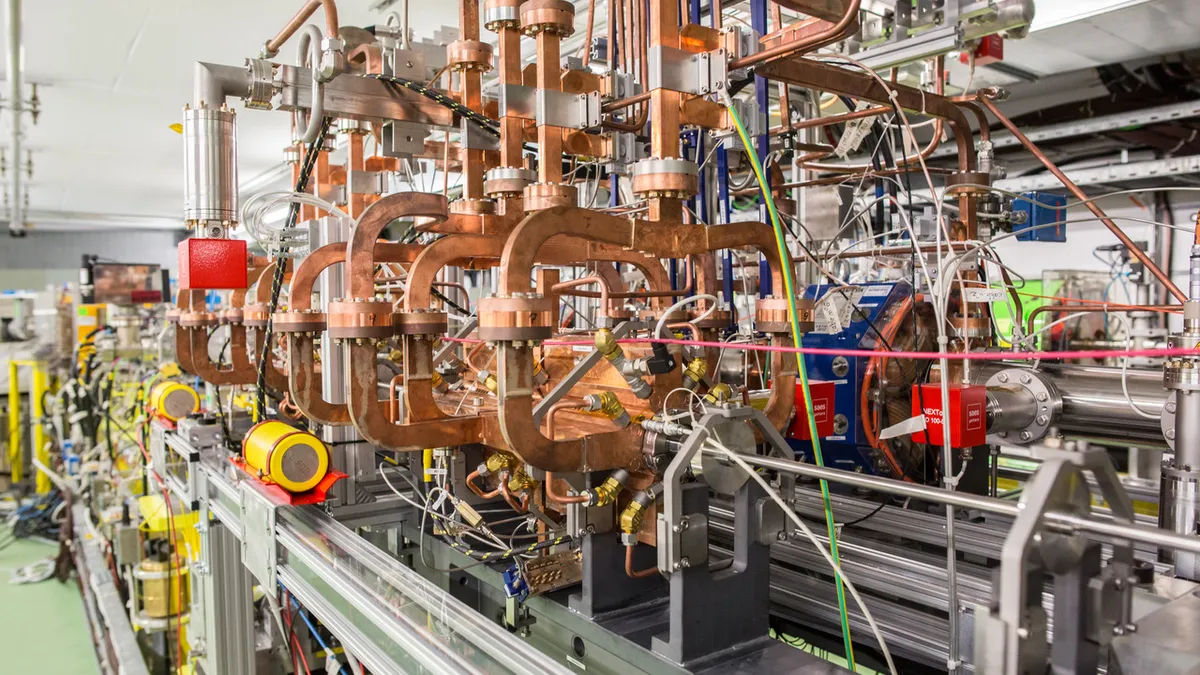
World’s largest particle physics laboratory backs push to transform cancer radiotherapy
Scientists, researchers and physicians will develop a machine to use high-speed particles to attack tumors deep inside the human body.
By Nick Paul Taylor • Nov. 29, 2022 -
Baxter recall of connected incontinence pads ranked by FDA as Class I event
The recall was assigned to the highest risk category as it could affect medically necessary devices, including insulin pumps and fetal monitors.
By Nick Paul Taylor • Nov. 28, 2022 -
Masimo’s pulse oximeter has no significant racial bias, study finds
The company said the result reflects its use of four additional signal processing engines that cut the impact of factors including skin pigmentation.
By Nick Paul Taylor • Nov. 28, 2022 -
Europe faces 2024 bottleneck in device reapprovals as submissions slow
While approval certificates for three-fourths of Europe’s medical devices will expire in 2024, a survey found no increase in the pace of submissions.
By Nick Paul Taylor • Nov. 28, 2022 -
Teleflex launches another round of restructuring
Rising costs are pushing the device maker to incur restructuring liabilities of $31 million to $40 million.
By Elise Reuter • Nov. 23, 2022 -
‘Tripledemic’ concerns spur FDA to issue emergency authorization for flu-COVID-19 combination test
Experts have warned “a viral hurricane is making landfall” as cases of RSV, influenza and COVID-19 rise simultaneously.
By Nick Paul Taylor • Nov. 23, 2022 -
Philips respirator recall reaches 260 reported deaths, FDA says
The agency has received 90,000 medical device reports related to foam problems with Philips’ recalled sleep apnea devices and ventilators.
By Elise Reuter • Updated Nov. 23, 2022 -
Medtronic lowers full-year forecast, citing drop in procedure growth
A slower-than-expected recovery in certain procedures, including TAVR and spinal cord stimulation, curbed quarterly profit and revenue.
By Elise Reuter • Nov. 22, 2022 -
State AGs urge Apple to protect consumers’ reproductive health data
Apps holding sensitive health data should be required to delete nonessential information and clearly inform consumers what information they share with parties such as law enforcement, the 10 state attorneys general said.
By Rebecca Pifer Parduhn • Nov. 22, 2022 -
Zimmer lands 510(k) clearance for 3D-printed cementless knee replacement
The new joint uses anatomical data in combination with 3D printing to try to directly mimic the architecture of human spongy bone.
By Nick Paul Taylor • Nov. 22, 2022 -
Apple earbuds show promise as hearing aids in clinical trial
The earbuds performed comparably to hearing aids in terms of speech perception in quiet environments.
By Nick Paul Taylor • Nov. 22, 2022 -
Q3 Earnings Wrap: Bumpy time for medtech with inflation, FX, supply woes
Macroeconomic pressures were a persistent theme for the industry, with workforce reductions a part of the fallout for some companies.
By Susan Kelly • Nov. 21, 2022 -
Apple Watch app for monitoring Parkinson’s symptoms earns FDA clearance
The technology has been validated in a clinical trial, positioning h2o to create an app that enables real-time sharing of Parkinson’s symptom data.
By Nick Paul Taylor • Nov. 21, 2022 -
How wearable devices with skin-like stretchability may pave way for better health tracking
An AI-enabled prototype based on the stretchable technology was more than 95% effective at correctly identifying electrocardiogram signals.
By Nick Paul Taylor • Nov. 21, 2022 -
Q&A
Friday Q&A: Embecta CEO talks BD spinoff, future of diabetes devices
Dev Kurdikar spoke with MedTech Dive about developing an insulin pump for patients with Type 2 diabetes and what’s next for the firm.
By Elise Reuter • Nov. 18, 2022 -
AdvaMed pushes Biden to use $52B chip program to support medtech supply chains
The group wants agencies to coordinate with the medtech industry to ensure investments are directed toward “technologies that power medical devices.”
By Nick Paul Taylor • Nov. 18, 2022