Medical Devices: Page 93
-
FDA opens consultation on tissue removal devices to reduce cancer risk
The agency is seeking feedback on draft guidance to help manufacturers of tissue containment systems reduce risk that cancerous tissue could leak during procedures.
By Nick Paul Taylor • June 22, 2022 -
Lack of clinical evidence 'major gap' in digital health: study
The researchers framed the low scores as evidence of “a major gap in health care technology,” adding that there is a “significant opportunity” for companies that differentiate themselves with a more rigorous approach.
By Nick Paul Taylor • June 22, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
ZimVie faces challenges in focus on slow-growing, 'highly competitive' spine market: JP Morgan
Still, as a standalone company, the Zimmer spinoff may benefit from tapping higher-growth sub-segments, according to analysts.
By Nick Paul Taylor • June 21, 2022 -
Senseonics lands CE mark for 6-month CGM implant, teeing up Q3 launch in Europe
Senseonics is looking to the longer-lasting implant to re-energize its fight in a market dominated by Abbott Laboratories and Dexcom.
By Nick Paul Taylor • June 17, 2022 -
Boston Scientific inks $230M deal to buy majority stake in M.I.Tech
M.I.Tech is a South Korean manufacturer of endoscopic and urologic medical devices. Analysts at BTIG said the deal complements Boston Scientific’s non-vascular stent portfolio and boosts its international presence.
By Nick Paul Taylor • June 16, 2022 -
Medtronic's Martha says supply chain woes may ease in second half
The CEO told a conference he’s working on plans to revamp pricing as inflation rises and developing a strategy for advancing robotic surgery.
By Nick Paul Taylor • June 16, 2022 -
User fee package goes to Senate with lab-developed test, OTC hearing aid provisions
The Senate HELP committee passed its version of the FDA user fee bill by a 13-9 vote. It includes an overhaul of diagnostic testing regulations and a requirement to create a category of over-the-counter hearing aids.
By Elise Reuter • June 15, 2022 -
ResMed to acquire German health software company MediFox for $1B
The acquisition continues a pattern of medical device companies buying software platforms. ResMed expects the deal to close by the end of the year.
By Ricky Zipp • June 14, 2022 -
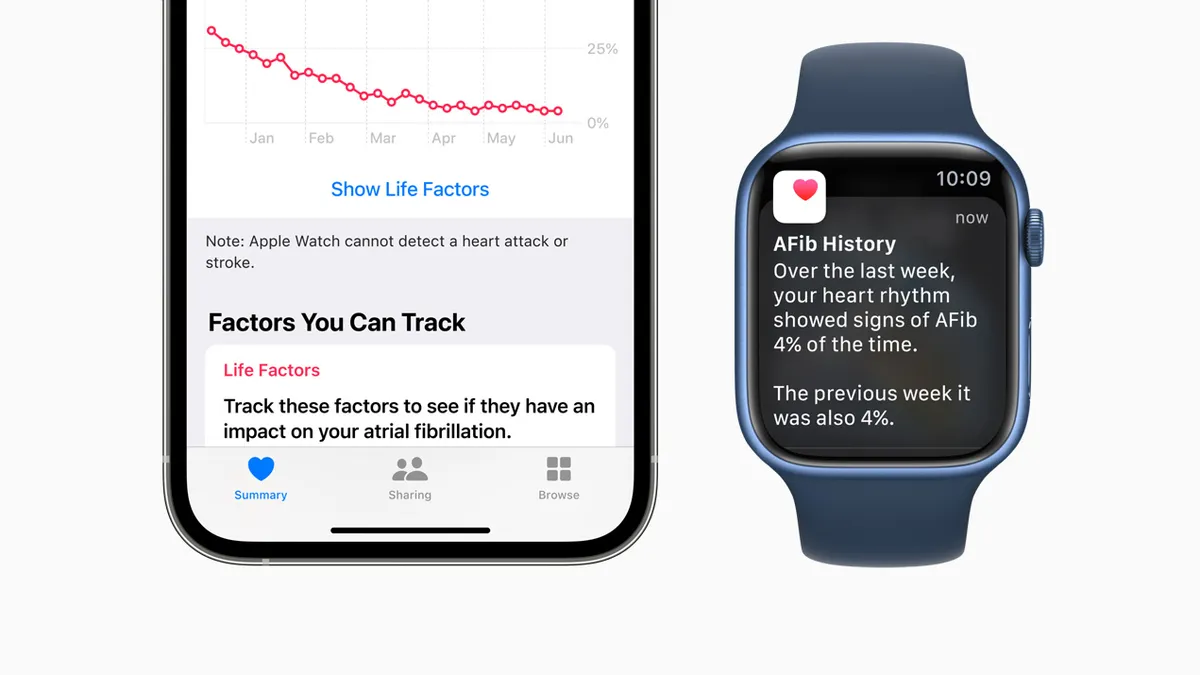
Apple Watch monitoring features for AFib, Parkinson's cleared by FDA
With the new feature, atrial fibrillation patients may have an easier way to track the frequency of the condition over time and see whether lifestyle changes may have positive effects.
By Nick Paul Taylor • June 14, 2022 -
Dräger's breathing system filter recall gets Class I label from FDA after ventilation obstruction
An obstruction in one of the company’s SafeStar 55 devices resulted in a patient being resuscitated after becoming hypoxic.
By Nick Paul Taylor • June 13, 2022 -
Medical device companies to curb spending as recession threat clouds longer-term outlook: analysts
The supply chain and inflation issues that have affected medical device companies in the first half of 2022 likely will persist through the rest of the year, according to RBC Capital Markets.
By Nick Paul Taylor • June 13, 2022 -
ResMed warns supply constraints may last 18 months after Philips completes recall of sleep-apnea devices
Analysts at William Blair expect ResMed to achieve “durable market share gains, bordering on permanent” because of Philips’ recall, which affects millions of sleep-apnea and ventilator devices.
By Nick Paul Taylor • June 10, 2022 -
UnitedHealth links CGM use in people with Type 2 diabetes to improved blood sugar control
J.P. Morgan analysts said they see the results as evidence that continuous glucose monitors have a role to play in support packages for people with Type 2 diabetes.
By Nick Paul Taylor • June 10, 2022 -
House passes FDA user-fee package, bolstering cybersecurity, clinical trial diversity for medical devices
By a 392-28 vote, the House of Representatives passed its version of the legislation, which would renew the Food and Drug Administration's ability to collect user fees for the next five years.
By Elise Reuter • June 9, 2022 -
Medtronic: No new patient injury, death reports since April letters warning of HVAD defect
The company’s HeartWare Ventricular Assist Device System received a Class I label from the FDA following two patient injuries and one death.
By Ricky Zipp • Updated June 14, 2022 -
Insulet Omnipod 5 release
Insulet targets competitive conversions as Omnipod 5 insulin pump nears full launch
The company said at a conference it aims to increase the rate of insulin pump use among people with Type 1 diabetes in the U.S.
By Nick Paul Taylor • June 9, 2022 -
GE Healthcare ventilator battery recall tied to over 1,500 complaints
Ventilators risk shutting down prematurely — cutting the flow of oxygen to patients — as backup batteries may run out of power earlier than expected.
By Nick Paul Taylor • Updated June 28, 2022 -
FDA mulls pilot program on alternative sterilization for medical devices
The proposed initiative is a response to global supply chain constraints and is intended to support sterilization supply chain resiliency, the agency said.
By Nick Paul Taylor • June 8, 2022 -
BD to acquire Parata for $1.53B as part of strategy to boost margins
The deal is expected to help Becton Dickinson reach its goal of a 25% operating margin by the end of 2025. J.P. Morgan analysts said it would be the most meaningful tuck-in deal since BD acquired Bard in 2017.
By Elise Reuter • June 7, 2022 -
Q&A
Dexcom CEO Sayer on G7 FDA submission, developing a 15-day sensor, M&A plans
Kevin Sayer spoke about the company's back-and-forth with the FDA on the G7 CGM system, possibly expanding from a 10-day to a 15-day sensor and on a report that Dexcom was in talks to buy Insulet.
By Ricky Zipp • June 7, 2022 -
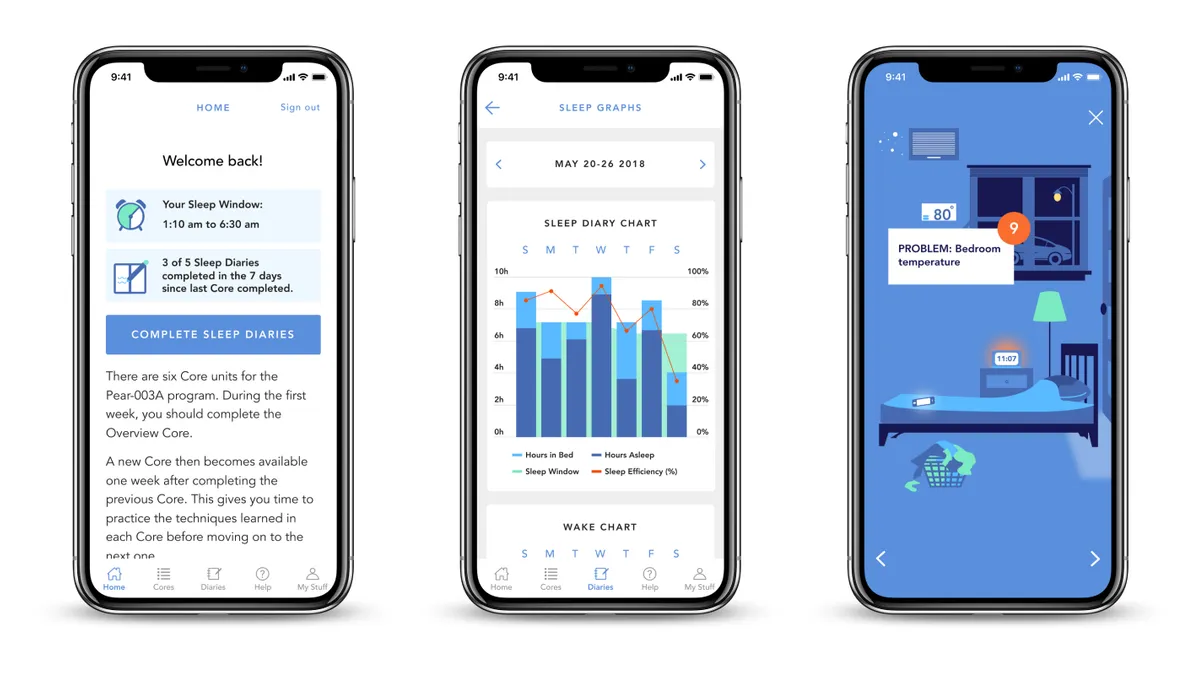
Pear sets out payer strategy as it seeks to quadruple prescriptions of digital therapeutics
Pear shared real-world data that linked its digital therapeutic for substance use disorder to an estimated $3,591 reduction in per-patient costs.
By Nick Paul Taylor • June 7, 2022 -
Abbott, Medtronic make case for key diabetes products in ADA presentations
Researchers at Abbott Laboratories used the event to highlight data on the FreeStyle Libre 3 continuous glucose monitor, while Medtronic arrived at the sessions with real-world results on the MiniMed 780G insulin pump.
By Nick Paul Taylor • June 6, 2022 -
Getinge's recall of 69,000 stents labeled as Class I event by FDA amid complaints, injuries
The recall relates to the separation of the balloon or catheter hub during the removal of the delivery system from the patient.
By Nick Paul Taylor • June 3, 2022 -
Medtronic expects diabetes unit's struggles to continue as rivals grow sales, launch products
The company forecasts revenues for the diabetes business to decrease 8% to 10% in the first quarter of its fiscal year and 6% to 7% for the full year.
By Ricky Zipp • June 1, 2022 -
BD's Pyxis medication dispenser gets fifth DHS cybersecurity alert in 5 years
The company said there are no known public exploits that specifically target a password vulnerability and that it's working to address the problem.
By Nick Paul Taylor • June 1, 2022