Medical Devices: Page 98
-
Panel warns against 'knee-jerk' rejection of globalization, calls for medical supply chain transparency
Instead of onshoring device manufacturing to stabilize supply chains, a congressionally-mandated committee has recommended more transparency, including disclosing manufacturing sites for all products and components.
By Nick Paul Taylor • March 7, 2022 -
FDA final guidance presses industry to be 'recall ready'
AdvaMed said the biggest concern with the earlier guidance was the potential for conflicting interpretations of whether an older regulation still applies to the initiation of recalls. The FDA has clarified the issue in the finalized guidance.
By Nick Paul Taylor • March 4, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Industry supports FDA's proposed quality system regulations, but says more time needed to adjust
"I think the potential benefit is likely to be realized very far downstream," Robert Phillips, vice president of quality and regulatory for Siemens Healthineers, said at the FDA's Wednesday meeting on QSR.
By Elise Reuter • March 3, 2022 -
75% of infusion pumps have cyber flaws, putting them at risk from hackers: study
An analysis of more than 200,000 infusion pumps, using crowd-sourced data supplied by healthcare organizations, found about half were susceptible to "critical" and "high" severity cybersecurity vulnerabilities.
By Greg Slabodkin • March 3, 2022 -
Boston Scientific touts early results of real-world Watchman FLX study
The device, which seals off the left atrial appendage, performed similarly in the analysis to a pivotal study, with 82% of patients experiencing no leak 45 days after implant. A competing Abbott product is expected to take market share.
By Elise Reuter • March 1, 2022 -
FDA's performance against MDUFA IV decision goal falls to new low
The agency's premarket approval target is set to slump, with the current figure for fiscal 2021 sitting almost 20% below the prior full-year low. FDA warned in September it may not "make good" on some MDUFA IV commitments.
By Nick Paul Taylor • March 1, 2022 -
Deep Dive
Medtech, hospitals on alert for cyberattacks after Russia's invasion of Ukraine
While cybersecurity threats to healthcare and medical devices have grown during the pandemic, the Russia-Ukraine conflict has raised the threat level, putting patient safety at risk.
By Greg Slabodkin • Feb. 28, 2022 -
HHS sets out plans to make medical device, diagnostics supply chains pandemic-proof
The U.S. Department of Health and Human Services wants to shore up the public health supply chain by investing in personal protective equipment, durable medical equipment and testing.
By Nick Paul Taylor • Feb. 28, 2022 -
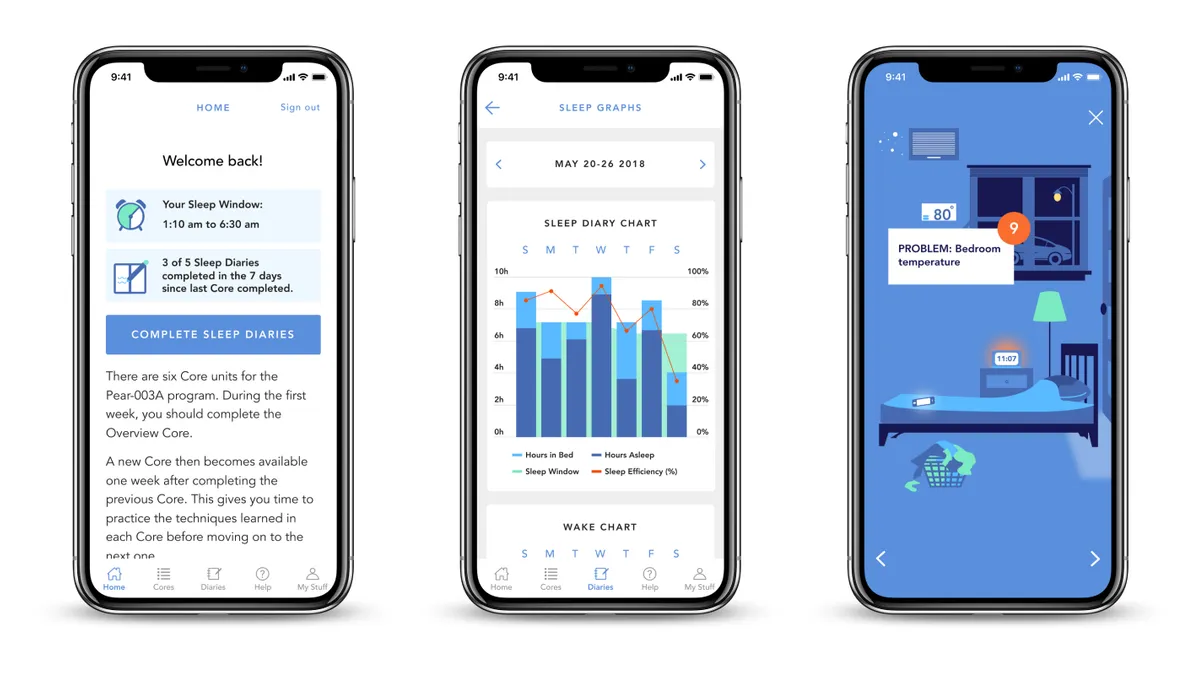
CMS code seen as major step toward reimbursement for digital therapeutics
Pear Therapeutics CEO Corey McCann said the agency's new HCPCS code for prescription digital behavioral therapy is an important milestone for getting digital therapeutics as a product category covered by more insurance plans.
By Elise Reuter • Feb. 25, 2022 -
Roche's Foundation Medicine gets FDA breakthrough device nod in latest designations
Breakthrough status was granted to Foundation Medicine's test to detect molecular residual disease in early-stage cancer after curative therapy. A liquid biopsy from Datar Cancer Genetics was also given the regulatory privileges.
By Nick Paul Taylor • Feb. 25, 2022 -
ResMed says it can't keep up with surging demand amid Philips recall
CEO Mick Farrell said ResMed continues to struggle to meet surging demand for its respiratory devices, in part because of shortages of semiconductors and other key materials.
By Elise Reuter • Feb. 24, 2022 -
Baxter hit with $18M SEC penalty for accounting improprieties
The U.S. Securities and Exchange Commission settled charges and levied a penalty against Baxter "for engaging in improper intra-company foreign exchange transactions" resulting in the net income misstatement.
By Nick Paul Taylor • Feb. 24, 2022 -
Diabetes tech leaders expect another year of growth, innovation as competition soars
MedTech Dive spoke with diabetes technology leaders about what to expect in 2022, the impact of new products and the increasingly competitive market.
By Ricky Zipp • Updated May 12, 2022 -
Smith & Nephew hires CEO from Siemens Healthineers, forecasts growth for 2022
The orthopaedics company's stock jumped 8% on the CEO news, even though its fourth-quarter revenue fell slightly short of the analyst consensus. Smith & Nephew is more optimistic about 2022 than rivals Stryker and Zimmer Biomet.
By Nick Paul Taylor • Feb. 23, 2022 -
What does a more aggressive M&A approach mean for J&J's devices unit?
After medical device deals took off last year, one of the industry's biggest companies could make a splash in the sector in 2022.
By Ricky Zipp , Elise Reuter • Feb. 22, 2022 -
FDA publishes proposed rule to align quality system requirements with international standards
The rule, which harmonizes U.S. good manufacturing practices with ISO standards, could save device companies hundreds of million of dollars by making it easier for those that comply with both standards, the agency says.
By Elise Reuter • Feb. 22, 2022 -
Baxter infusion pump recall labeled Class I by FDA
The recall labeling on Friday follows a safety notification issued by the agency in February, which warned that "use of the affected products may cause serious adverse events."
By Elise Reuter • Updated March 14, 2022 -
Medtronic's Q3 revenue misses estimates as omicron curbs procedures
CEO Geoff Martha on Tuesday told investors the COVID-19 resurgence, which peaked in the final weeks of January, curbed procedure volumes. He expects them to recover in March and April.
By Greg Slabodkin • Feb. 22, 2022 -
FDA calls device manufacturing expert meeting to discuss quality system harmonization
After the agency began transitioning its Quality System Regulation in 2018, the public may finally get to see the proposed rule in late February or early March.
By Nick Paul Taylor • Feb. 18, 2022 -
Q&A
Medtronic talks diabetes group's FDA warning letter, new products, supply chain constraints
Ali Dianaty, VP of product innovation and operations for Medtronic Diabetes, spoke to MedTech Dive about the warning letter's impact on product reviews, creating insulin pump patches and navigating the pandemic.
By Ricky Zipp • Feb. 18, 2022 -
Baxter begins integrating Hillrom, faces supply chain pressures
The recent omicron surge put a damper on the company's margins, as shipping costs and healthcare staff shortages rose. Baxter expects these problems to abate in the short term and sees a long-term boost from the Hillrom acquisition.
By Elise Reuter • Feb. 17, 2022 -
EU issues guidance on high-risk IVDs, surveillance of legacy medical devices
The documents outline how notified bodies can meet In Vitro Diagnostic Medical Devices Regulation requirements to verify product batches of high-risk class D diagnostics, and manufacturer rules on quality management systems.
By Nick Paul Taylor • Feb. 17, 2022 -
Diagnostics M&A expected to be strong in 2022 after dealmaking took off last year
Respondents to KPMG's survey predict that deal volumes and values for the diagnostics space will jump again this year as companies are ready to spend more of their COVID-19 cash.
By Nick Paul Taylor • Feb. 16, 2022 -
Califf's confirmation comes as FDA, medtechs skirmish over regulations
Industry groups, including AdvaMed, congratulated Robert Califf on his confirmation as FDA commissioner. But they're still negotiating with the agency over medical device user fees and lab-developed tests.
By Jonathan Gardner • Updated Feb. 16, 2022 -
Latest Essure data show more patients had device removed
According to the latest results from two FDA postmarket studies, the number of people who had the birth control implant removed or experienced chronic pain increased.
By Nick Paul Taylor • Feb. 15, 2022